Fundamentals of Aquatic Veterinary Medicine
Здесь есть возможность читать онлайн «Fundamentals of Aquatic Veterinary Medicine» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Fundamentals of Aquatic Veterinary Medicine
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Fundamentals of Aquatic Veterinary Medicine: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Fundamentals of Aquatic Veterinary Medicine»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Covers the competencies necessary to assure the highest quality of aquatic veterinary services Fundamentals of Aquatic Veterinary Medicine
Fundamentals of Aquatic Veterinary Medicine — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Fundamentals of Aquatic Veterinary Medicine», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
1.3.2.11 Conductivity, Salinity, Total Dissolved Solids
Conductivity is a measure of water’s capability to pass an electrical current. This ability is directly related to the concentration of ions in the water (US Environmental Protection Agency, 2016). These conductive ions come from dissolved salts and inorganic materials such as alkalis, chlorides, sulfides and carbonate compounds (Miller et al ., 1988). Compounds that dissolve into ions are also known as electrolytes (Sommer and Spitzer, 2004). The more ions that are present, the higher the conductivity of water. Likewise, the fewer ions that are in the water, the less conductive it is. A sudden increase in pond conductivity can indicate pollution. Agricultural runoff or a sewage leak will increase conductivity due to the additional chloride, phosphate, and nitrate ions.
Salinity is a measure of the total concentration of dissolved solids, usually in parts per thousand (ppt). Anions (negatively charged) are chloride, sulfate, bicarbonate and bromide. Cations (positively charged) are sodium, magnesium, calcium, potassium, and strontium. Sodium and chloride are the major solids. Because the electrolytes form ionic particles as they dissolve, each with a positive and negative charge, salinity is a strong contributor to conductivity. The concentration of water is as follows:
Freshwater: less than 2 ppt
Brackish water: 2–16 ppt
Saltwater: 35 ppt.
Most aquatic organisms can only tolerate a specific salinity range (Clean Water Team, 2002). The physiological adaptation of each species is determined by the salinity of its surrounding environment. Salinity tolerances depend on the osmotic processes within an organism. Fish and other aquatic life that live in fresh water (low salinity) are hyperosmotic (cells have a high ability to eliminate water and retain ions). On the other side of the spectrum, saltwater (high salinity) organisms are hypo‐osmotic and maintain a lower internal ionic concentration than seawater. Most species of fish are stenohaline, exclusively freshwater, or exclusively saltwater (Myers, 1949). However, there are a few organisms that can adapt to a range of salinities. These euryhaline organisms can be anadromous, catadromous or true euryhaline. Anadromous organisms live in saltwater but spawn in freshwater. Catadromous species are the opposite – they live in freshwater and migrate to saltwater to spawn (Myers, 1949). True euryhaline species can be found in saltwater or freshwater at any point in their life cycle (Myers, 1949). Estuarine organisms are true euryhaline.
The sum of all ion particles that are smaller than 2 microns (0.0002 cm) is the total dissolved solids. This includes all the disassociated electrolytes that make up salinity concentrations, as well as other compounds such as dissolved organic matter. Freshwater aquasystems should have less than 2000 mg/l of total dissolved solids and most water sources should have much less than that (American Public Health Association et al ., 2017).
1.3.2.12 Light, Color, Vibration and Noise
Aquasystem water color influences microplankton growth, which can affect the stability of the water chemistry. Water clarity can affect fish. If fish that prefer turbid waters (e.g., bullhead, catfish, walleye) are cultured in relatively clear water they will experience stress; survival and growth will be adversely affected. Intensive aquaculture systems, particularly recirculating systems, utilize equipment such as aerators, air and water pumps, blowers, and filtration systems that inadvertently increase vibration and noise levels in fish culture tanks (see also vibration and noise in Chapter 10). Field and laboratory studies have shown that fish behavior and physiology can be negatively impacted by intense sound. Chronic exposure to aquaculture production noise could therefore cause increased stress, reduced growth rates, and cold feed conversion efficiency, and decreased survival. In the wild, geological and geophysical exploration, pile driving, drilling, dredging, and vessel traffic all produce manmade noise and vibrations, which may have negative effects on native fauna, which may range from physiological and behavioral effects to physical damage.
1.3.2.13 Greenhouse Gases and Climate Change
Elevation of carbon dioxide in the atmosphere leads to acidification of our water. This change affects all aquatic life. Millions of dollars are lost each year on decreased growth and increased mortality due to large fluctuations in the pH and temperatures of the water. Elevation in the CO 2levels of the atmosphere push the chemical reaction:
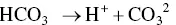
to move more toward a disassociation of water molecules and increase the HCO 3 –. In the ocean, this fluctuation is well buffered by salt water, but in coastal areas and rivers the addition of runoff increases the nitrogen, carbon and Ca 2 +, creating large fluctuations in the pH. Climate change has resulted in an increase of the ocean temperatures, changes in mixing and increased intensity of storms globally. These changes, together with large fluctuations in pH, result in large hypoxic zones and the inability of fish to acclimate to them.
Fish in estuaries and coastal areas are affected the most by acidification due to the increased fluctuation of the pH. This fluctuation is exaggerated by nutrient runoff, coastal upwelling, and atmospheric pollution. Fish have been shown to be able to regulate their pH within days, but they rely on the ability to move the CO 2into the lower partial pressure of carbon dioxide (pCO 2) of the aquatic environment. As atmospheric CO 2increases, fish can compensate metabolically and regulate their pH but still show elevated levels of the CO 2in their extracellular fluids. Studies have shown that this elevated level of CO 2can cause problems with osmoregulation. In addition, studies have shown this increased level of CO 2can result in increased otolith size in clownfish, Atlantic cod and pollock (Heuer and Grosell, 2014).
The increased CO 2in the plasma has been shown to affect the metabolic rate and the formation of CaCO 3 +. This occurs when HCO 3 –is secreted into the intestinal lumen. This secretion stimulates the secretion of CO 2. This combination precipitates the Ca2 +from the seawater that has been ingested. These then combine to form CaCO 3 +. The higher PCO 2in the plasma has been proven to increase these secretions in midshipmen and toadfish. CaCO 3 +is important for osmoregulation in fish by influencing the osmotic pressure in the luminal cavity. Changes in the amount of CaCO 3 +has the potential to decrease the osmotic balance (Heuer and Grosell, 2014).
Elevations in atmospheric CO 2have been shown to decrease calcification rates in the carbonate equation:
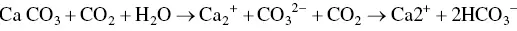
Decreases in calcification rates affect coral reefs, planktonic microalgae, coccolithophores, phytoplankton, and all shellfish. Coral reef growth has been shown to decline due to the decrease of calcification, with a predicted decrease of 21–40% over the period of 1880–2065 (Turley et al ., 2006). This decline is also dependent on the temperature of the water and other available nutrients. Oysters and abalone have been the focus of many studies in regards to acidification. These organisms have been shown to have a decreased rate of survival at the larval stage due to lack of calcification of the shell and decreased ability to ingest nutrients. The shell of mollusks is produced throughout all its life stages. During the larval stages, the shell is made from deposition of amorphous calcium carbonate, which then forms into a crystalline aragonite. Both these stages of shell development are more soluble in a low pH. Studies have shown that certain levels of pH will cause holes to form in the shells (Wessel et al ., 2018).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Fundamentals of Aquatic Veterinary Medicine»
Представляем Вашему вниманию похожие книги на «Fundamentals of Aquatic Veterinary Medicine» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Fundamentals of Aquatic Veterinary Medicine» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.