James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
For the purposes of pricing, crude oil is generally classified based on the API gravity and sulfur content. For example, light crude oil has low density, low viscosity (there are no exact numbers assigned to this, because the classification is more practical and theoretical), and low sulfur content, making it easier to transport and refine and, therefore, more expensive to purchase. Sweet crude oil has a sulfur content less than 0.5% by weight and is usually (but not always) light crude oil, making it much easier to refine in a way that would meet environmental standards in developed countries - and making it more expensive.
A light crude oil is generally one with an API gravity of less than approximately 40 – for example, Brent crude oil has an API gravity on the order of 38 to 39°. Sweet crude is preferable to sour crude oil because it is also (like light crude) more suited to the production of the most valuable refined products. On the other hand, heavy crude oil has high density, high viscosity, and high sulfur content making it more difficult to transport and refine and cheaper to purchase. Typically, sour crude oil has a sulfur content above 0.5% by weight and is usually heavy crude oil , making it cheaper to purchase but more expensive to refine.
Heavy crude oil will typically have an API gravity of 20 or less - the higher the API gravity, the lower the density. Heavy crude oil is harder to handle (it is too thick to pump easily through pipelines unless diluted with light crude) and is more expensive to refine to produce the most valuable crude oil products such as naphtha (gasoline), kerosene, diesel fuel, and aviation fuel.
Because there are so many different varieties and grades of crude oil, buyers and sellers have found it easier to refer to a limited number of reference, or benchmark, crude oils. Other varieties are then priced at a discount or premium, according to their quality. Thus, crude oil is priced in terms of regional blends, each with different characteristics. Of these, certain blends are followed by traders, as they most reflect the overall value of oil, and therefore affect the way different blends are priced. These are essentially like a Consumer Price Index for different types of oil. Approximately 160 different types of crude are traded around the world; the four primary benchmarks, of which these are priced internationally are (i) Brent blend crude oil, (ii) West Texas intermediate (WTI) crude oil, (iii) Dubai crude oil, and (iv) the OPEC Basket crude oil.
The Brent crude oil blend is based on the prices of Brent crude, which is a light, sweet crude oil and is actually a combination of crude oil from 15 different oil fields in the Brent and Ninian systems located in the North Sea. The API gravity is 38.3° (making it a “light” crude oil, but not quite as “light” as West Texas Intermediate crude oil), while it contains approximately 0.37% by weight sulfur (making it a sweet crude oil, but again slightly less sweet than West Texas Intermediate crude oil). The Brent blend is ideal for making gasoline and middle distillates, both of which are consumed in large quantities in Northwest Europe, where Brent blend crude oil is typically refined. However, if the arbitrage between Brent and other crude oils, including WTI, is favorable for export, Brent has been known to be refined in the United States (typically the East Coast or the Gulf Coast) or the Mediterranean region. Brent blend, like West Texas Intermediate crude oil, production is also on the decline, but it remains the major benchmark for other crude oils in Europe or Africa.
Beta Decay
Beta decay ( β -decay) is a type of radioactive in which a beta particle (a fast energetic electron or positron) is emitted from an atomic nucleus which transforms the original nuclide to an isobar of that nuclide.
By definition, an isobar is an atom (nuclide) of different chemical elements that have the same number of nucleons. Correspondingly, an isobar differs in atomic number (or the number of protons) but has the same mass number. An example of a series of isobars is 40S, 40Cl, 40Ar, 40K, and 40Ca.
Thus, the beta decay of a neutron transforms it into a proton by the emission of an electron accompanied by an antineutrino or, conversely, a proton is converted into a neutron by the emission of a positron with a neutrino in the positron emission reaction. Neither the beta particle nor its associated (anti-)neutrino exist within the nucleus prior to beta decay, but are created in the decay process. By this process, unstable atoms obtain a more stable ratio of protons to neutrons. The probability of a nuclide decaying due to beta and other forms of decay is determined by the nuclear binding energy.
Thus, the two types of beta decay are known as beta minus and beta plus . In the former [beta minus decay (β −decay)], a neutron is converted to a proton, and the process creates an electron and an electron antineutrino, while in beta plus decay (β +decay, also known as positron emission), a proton is converted to a neutron and the process creates a positron and an electron neutrino.
An example of electron emission (β −decay) is the decay of carbon-14 (1 4C) into nitrogen-14 ( 14N) with a half-life on the order of 5,730 years:
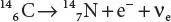
In this form of decay, the original element becomes a new chemical element (nuclear transmutation) and the new element has an unchanged mass number ( A ) but anatomic number ( Z ) that is increased by one. As in all nuclear decays, the decaying element (in this case 14 6C) is the parent nuclide , while the resulting element (in this case 14 7N) is known as the daughter nuclide .
Another example is the decay of hydrogen-3 ( 3H, tritium) into helium-3 ( 3He) with a half-life on the order of 12.3 years:
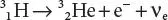
An example of positron emission (β +decay) is the decay of magnesium-23 ( 23Mg) into sodium-23 ( 23Na) with a half-life on the order of 11.3 seconds:
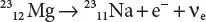
Beta+ decay (β +decay) also results in nuclear transmutation, with the resulting element having an atomic number that is decreased by one.
See also: Alpha Decay, Alpha Particle, Nuclear Energy.
Beta Particle
A beta particle is emitted from the nucleus of a radioactive atom with a wide range of energies up to some maximum value. When a beta particle is emitted that is below the maximum value, the neutrino carries away the rest of the energy.
Like the alpha particles, beta particles, like alpha particles, lose energy by ionization and excitation, but because of their small mass (1/7,300 of an alpha particle) and lower charge (1/2 of that of an alpha particle), the interactions take place at less frequent intervals. Therefore, the beta particles do not produce as many ion pairs per centimeter of path as alpha particles, and thus, have a greater range in matter which depends on the energy of the particle and the composition of the material.
See also: Alpha Decay, Alpha Particle, Nuclear Energy.
Bioalcohol
Biologically produced alcohols, such as ethanol, propanol and butanol, are produced by the action of microorganisms and enzymes through the fermentation of starches, sugars, or cellulose. Alcohol fuels are produced by fermentation of sugars derived from corn, molasses, sugar beets, sugar cane, wheat, as well as potato and fruit waste.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.