James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Table A-19Amines (olamines) used for gas processing.
| Olamine | Formula |
|---|---|
| Ethanolamine (monoethanolamine) (MEA) | HOC 2H 4NH 2 |
| Diethanolamine (DEA) | (HOC 2H 4) 2NH |
| Triethanolamine (TEA) | (HOC 2H 4) 3N |
| Diglycolamine (hydroxyethanolamine) (DGA) | H(OC 2H 4) 2NH 2 |
| Diisopropanolamne (DIPA) | (HOC 3H6) 2NH |
| Methyldiethanolamine (MDEA) | (HOC 2H 4) 2NCH 3 |
In the process, the sour gas is run through a tower, which contains the olamine solution. There are two principal amine solutions used, monoethanolamine (MEA) and diethanolamine (DEA). Either of these compounds, in liquid form, will absorb sulfur compounds from natural gas as it passes through. The effluent gas is virtually free of sulfur compounds, and thus loses its sour gas status. Like the process for the extraction of natural gas liquids and glycol dehydration, the amine solution used can be regenerated for reuse.
As currently practiced, acid gas removal processes involve the selective absorption of the contaminants into a liquid, such as an olamine ( Table A-19), which is passed countercurrent to the gas. Then, the absorbent is stripped of the gas components (regeneration) and recycled to the absorber. The process design will vary and, in practice, may employ multiple absorption columns and multiple regeneration columns.
Liquid absorption processes (which usually employ temperatures below 50°C (120°F) are classified either as physical solvent processes or chemical solvent processes . The former processes employ an organic solvent, and absorption is enhanced by low temperatures, or high pressure, or both. Regeneration of the solvent is often accomplished readily. In chemical solvent processes, absorption of the acid gases is achieved mainly by use of alkaline solutions such as amines or carbonates. Regeneration (desorption) can be achieved by the use of reduced pressure and/or high temperature, whereby the acid gases are stripped from the solvent.
Regeneration of the solution leads to near complete desorption of carbon dioxide and hydrogen sulfide. A comparison between monoethanolamine, diethanolamine, and diisopropanolamine shows that monoethanolamine is the cheapest of the three but shows the highest heat of reaction and corrosion; the reverse is true for diisopropanolamine.
The processes using ethanolamine and potassium phosphate are now widely used. The ethanolamine process, known as the Girbotol process, removes acid gases (hydrogen sulfide, and carbon dioxide) from liquid hydrocarbons as well as from natural and from refinery gases. The Girbotol process uses an aqueous solution of ethanolamine (H 2NCH 2CH 2OH) that reacts with hydrogen sulfide at low temperatures and releases hydrogen sulfide at high temperatures. The ethanolamine solution fills a tower called an absorber through which the sour gas is bubbled. Purified gas leaves the top of the tower, and the ethanolamine solution leaves the bottom of the tower with the absorbed acid gases. The ethanolamine solution enters a reactivator tower where heat drives the acid gases from the solution. Ethanolamine solution, restored to its original condition, leaves the bottom of the reactivator tower to go to the top of the absorber tower, and acid gases are released from the top of the reactivator.
The chemistry can be represented by simple equations for low partial pressures of the acid gases:
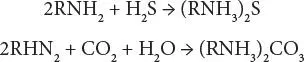
At high acid gas partial pressure, the reactions will lead to the formation of other products:
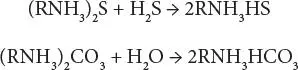
The reaction is extremely fast, the absorption of hydrogen sulfide being limited only by mass transfer; this is not so for carbon dioxide.
See also: Gas Cleaning, Gas Processing, Gas Treating.
Ammonia
Ammonia is a valuable chemical that is often produced from renewable sources (biomass) that contain nitrogen. It is recoverable from both the liquid and gas streams and can be readily separated from the liquid, by an ammonia still. The fixed salts must be treated with lime or caustic in a lime leg. The gaseous ammonia may be absorbed with sulfuric acid to produce ammonium sulfate, processed to anhydrous ammonia, or destroyed by combustion.
The standard procedure for the manufacture of ammonium sulfate from a gas stream involves several steps. The gas is first cooled to approximately 32°C (90°F) in an appropriate condensation system. Any tar, which is a very troublesome contaminant of ammonium sulfate (and vice versa), condenses and, in addition, much of the water containing approximately 25% of the ammonia (NH 3), primarily as ammonium (NH 4+) salts, also condenses. This water is rendered basic (lime treatment), thereby converting the ammonium ion to ammonia, which is recovered by being stripped off in a lime still and placed back in the coal gas stream. The coal gas stream is heated to above its dew point (approximately 65°C; 150°F), and the ammonia is adsorbed in 5 to 10% sulfuric acid solution contained in a lead-lined saturator at a temperature of 50 to 60°C (120 to 140°F); ammonium sulfate crystals precipitate from the sulfuric acid solution.
See also: Petrochemicals.
Anaerobic Digestion
Anaerobic digestion is the decomposition of biological wastes by microorganisms, usually under wet conditions, in the absence of air (oxygen), to produce a gas comprising mostly methane and carbon dioxide.
Anaerobic digestion of animal waste is the primary cause of odors, solids buildup and many diseases in swine, dairy, and poultry facilities, processing plants, municipal waste systems, and septic systems. Animal waste concentrated in pits under slatted floors or collected in holding tanks or lagoons has the natural tendency to involve an anaerobic process. Anaerobic digestion occurs when the anaerobic microbes are dominant over the aerobic microbes. Anaerobic microbes will naturally become dominant in pits or lagoons because of the lack of oxygen in solutions containing heavy concentrations of animal waste, which results in a high biological oxygen demand (BOD). These microbes feed on the animal waste at the bottom of the pits and lagoons. As they digest waste, large amounts of toxic gases are released due to the digestion processes common to the anaerobic microbes.
The anaerobic digestion o f animal waste can be changed to aerobic digestion by proper applications of beneficial aerobic microbes in a highly concentrated form. If aerobic microbes are introduced into an environment that is lacking oxygen, they will begin to build oxygen into this environment as long as they survive and reproduce. Aerobes have the ability to do this in a liquid media or in the soil as long as there is an adequate moisture and food source for them to feed on and reproduce. The process is a multi-stage biological treatment process whereby bacteria, in the absence of oxygen, decompose organic matter to carbon dioxide, methane, and water ( Table A-20).
Table A-20Schematic of the anaerobic digestion process.
| Feedstock | Products | Products | Products |
|---|---|---|---|
| Carbohydrates | Sugars | Carbon acids | Methane |
| Fats | Fatty acids | Alcohols | Carbon dioxide |
| Proteins | Amino acids | Hydrogen | |
| Carbon dioxide | |||
| Ammonia |
In this way, the waste sludge is stabilized and the obnoxious odor is removed. The process can, however, be described adequately and simply as occurring in two stages, involving two different types of bacteria. The process occurs in the absence of air, the decomposition in this case is caused not by heat but by bacterial action. In the first stage, the organic material present in the feed sludge is converted into organic acids (also called volatile fatty acids) by acid forming bacteria. In the second stage, these organic acids serve as the substrate (food) for the strictly anaerobic methane-producing bacteria, which converts the acids into methane and carbon dioxide.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.