James G. Speight - Encyclopedia of Renewable Energy
Здесь есть возможность читать онлайн «James G. Speight - Encyclopedia of Renewable Energy» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Encyclopedia of Renewable Energy
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Encyclopedia of Renewable Energy: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Encyclopedia of Renewable Energy»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Written by a highly respected engineer and prolific author in the energy sector, this is the single most comprehensive, thorough, and up-to-date reference work on renewable energy.
Encyclopedia of Renewable Energy: Audience
Encyclopedia of Renewable Energy — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Encyclopedia of Renewable Energy», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Alloys of tin and lead (Sn+ Pb) shall be avoided as welding material. All bronze parts shall be brass or stainless steel. Steel fuel lines shall be replaced by nylon tube (Nylon 11). Fuel filters used for gasoline are not recommended for the many alcohols. The internal element collapses after the glue that bonds it together is softened by the alcohol. Special filters are necessary. Also, due to the higher flows, filters have to be bigger. The filter body must be made of nylon or Teflon.
See also: Alcohols, Butanol, Ethanol, Methanol, Propanol.
Alcohols – From Waste
Alcohols can be made from organic materials by fermentation, and there is the potential for the production of alcohols from organic waste. Historically, the production of methanol, ethanol, and higher molecular alcohols from syngas has been known since the beginning of the 20 thcentury. There are several processes that can be used to make mixed alcohols from synthesis gas including iso-synthesis, variants of Fischer-Tropsch synthesis, oxo-synthesis involving the hydroformylation of olefins, and homologation of methanol and lower molecular weight alcohols to make higher alcohols. In the context of the Fischer-Tropsch process, depending on the process and its operating conditions, the most abundant products are usually methanol and carbon dioxide, but methanol can be recycled to produce higher molecular weight alcohols.
With the development of various gas-to-liquid processes, it was recognized that higher alcohols were by-products of these processes when catalysts or conditions were not optimized. Modified Fischer-Tropsch (or methanol synthesis) catalysts can be promoted with alkali metals to shift the products toward higher alcohols. Synthesis of higher molecular weight alcohols is optimal at higher temperatures and lower space velocities compared to methanol synthesis and with a ration of hydrogen/carbon monoxide ratio of approximately 1 rather than 2 or greater.
In the process, the feedstock enters the process and is converted to synthesis gas with the desired carbon monoxide/ hydrogen ratio, which is then reacted, in the presence of a catalyst, into methanol (CH 3OH), ethanol (CH 3CH 2OH), and higher molecular weight alcohols.
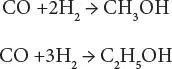
Thus,
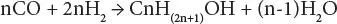
Stoichiometry suggests that the carbon monoxide/hydrogen ratio is optimum at 2, but the simultaneous presence of water-gas shift leads to an optimum ratio closer to 1.
As in other synthesis gas conversion processes, the synthesis of higher molecular weight alcohols generates significant heat and an important aspect is choice of the proper reactor to maintain even temperature control which then maintains catalyst activity and selectivity. In fact, the synthesis of higher molecular weight alcohols is carried out in reactors similar to those used in methanol and Fischer-Tropsch synthesis. These include shell and tube reactors with shell-side cooling, trickle-bed, and slurry bed reactors.
Catalysts for the synthesis of higher molecular weight alcohols generally fall mainly into four groups: (1) modified high pressure methanol synthesis catalysts, such as alkali-doped ZnO/Cr 2O 3, (2) modified low pressure methanol catalysts, such as alkali-doped Cu/ZnO and Cu/ZnO/Al 2O 3, (3) modified Fischer-Tropsch catalysts, such as alkali-doped CuO/CoO/Al2O3, and (4) alkali-doped sulfides, such as mainly molybdenum sulfide (MoS 2).
The catalytic synthesis process makes several different alcohols depending, in part, on residence time in the reactor and the nature of the catalyst. The alcohols can be separated by distillation and dried to remove water.
A further aspect of the waste-to-alcohols concept is the use of a plasma field (http://www.fuelfrontiers.com/technology.htm) in which temperatures are reputed (but not yet proved) to reach 30,000°C (54,000°F). The feedstock can be materials such as waste coal, used tires, wood wastes, raw sewage, municipal solid wastes, biomass, discarded roofing shingles, coal waste ( culm ), and discarded corn stalks. The plasma field breaks down the feedstock into their core elements in a clean and efficient manner.
See also: Alcohols.
Alcohols – Production
Alcohols can be produced from a variety of feedstocks. For example, ethanol is produced from organic feedstocks, such as biomass, by a fermentation process. However, recent efforts have focused on the production of ethanol from waste materials using a gasification process. The overall process consists of a thermochemical conversion of synthesis gas which is then converted to higher molecular weight alcohols by a catalytic process, after which high purity ethanol is separated by distillation. The process is similar to the methanol and gas-to-liquid process. The key differentiating factors are the catalysts and their operating parameters.
Alcohols can be made directly from organic feedstocks (such as sugars and sugar derivatives) by fermentation or indirectly by the production of synthesis gas, and there is the potential for the production of alcohols from organic waste.
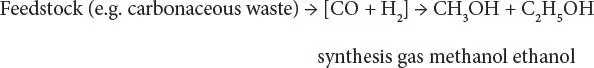
Historically, the production of methanol, ethanol, and higher molecular alcohols from synthesis gas has been known since the beginning of the 20 thcentury. There are several processes that can be used to make mixed alcohols from synthesis gas including iso-synthesis, variants of Fischer-Tropsch synthesis, oxo-synthesis involving the hydroformylation of olefins, and homologation of methanol and lower molecular weight alcohols to make higher alcohols. In the context of the Fischer-Tropsch process, depending on the process and its operating conditions, the most abundant products are usually methanol and carbon dioxide, but methanol can be recycled to produce higher molecular weight alcohols.
With the development of various gas-to-liquid processes, it was recognized that higher alcohols were by-products of these processes when catalysts or conditions were not optimized. Modified Fischer-Tropsch (or methanol synthesis) catalysts can be promoted with alkali metals to shift the products toward higher alcohols. Synthesis of higher molecular weight alcohols is optimal at higher temperatures and lower space velocities compared to methanol synthesis and with a hydrogen/carbon monoxide ratio of approximately 1 rather than 2 or greater.
In the process, the feedstock enters the process and is converted to synthesis gas with the desired carbon monoxide/ hydrogen ratio, which is then reacted, in the presence of a catalyst, into methanol (CH 3OH), ethanol (CH 3CH 2OH), and higher molecular weight alcohols.
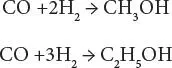
Thus,
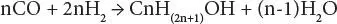
Stoichiometry suggests that the carbon monoxide/hydrogen ratio is optimum at 2, but the simultaneous presence of water-gas shift leads to an optimum ratio closer to 1.
As in other synthesis gas conversion processes, the synthesis of higher molecular weight alcohols generates significant heat and an important aspect is choice of the proper reactor to maintain even temperature control which then maintains catalyst activity and selectivity. In fact, the synthesis of higher molecular weight alcohols is carried out in reactors similar to those used in methanol and Fischer-Tropsch synthesis. These include shell and tube reactors with shell-side cooling, trickle-bed, and slurry bed reactors.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Encyclopedia of Renewable Energy»
Представляем Вашему вниманию похожие книги на «Encyclopedia of Renewable Energy» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Encyclopedia of Renewable Energy» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.