1 ...8 9 10 12 13 14 ...17 A subset of dilutions is selected for plating on a microbiological medium suitable for sustaining the targeted population. The extent of dilution and selection of the dilutions to be plated depends greatly on the analyst’s expectations of the size of microbial population in the food. The larger the population, the greater the degree of dilution required. An example of a dilution scheme is shown in Figure 2.3.
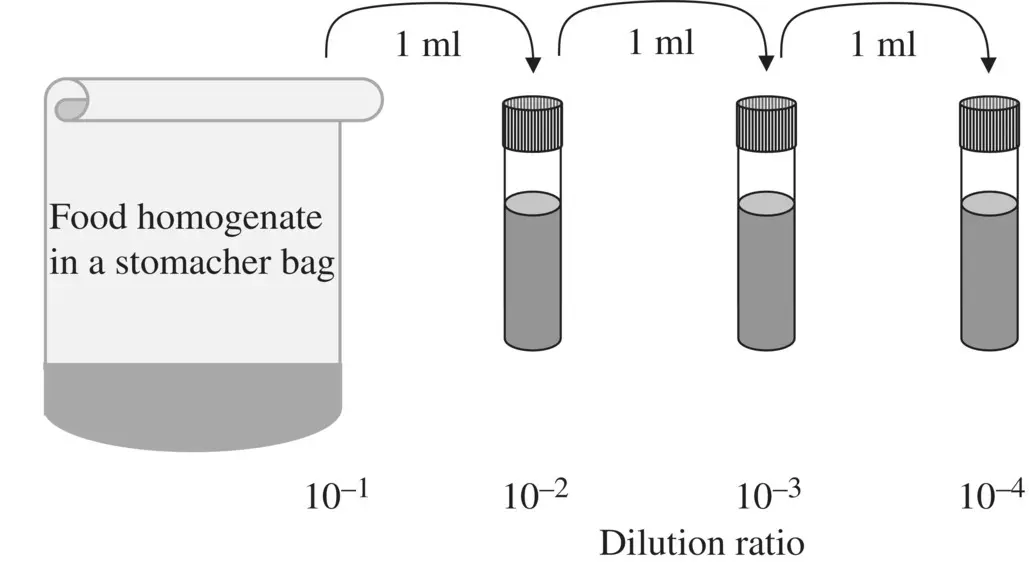
Figure 2.3 Decimal dilution of food homogenate.
Food microbiota include pre‐harvest contaminants as well as microorganisms introduced from the processing environment. Assessing the microbial load and variety of microorganisms in the factory environment provides insight into the quality and safety of the finished product produced in the facility. Consequently, the processing environment is often sampled, and results of analysis are carefully considered by the facility management. Samples are often taken from floors, drains, and equipment surfaces, particularly those that are food contact surfaces. Refrigerators and other storage sites also should be sampled frequently. The sampling procedure depends on the nature of the site, degree of contamination, and the microbiological information sought. There are two broad categories of samples that are commonly taken from food processing environment: surface and air samples.
Surfaces in processing facilities are commonly designated by their likelihood to cause food contamination. Food contact surfaces (Zone 1) are important sites that are often considered during environmental sampling. A food contact surface could be part of a piece of equipment, packing material, storage tank, ripening room, conveyer belt, or any other item that is expected to touch the food item. Zone 2 is an area where surfaces do not directly contact food but are usually located in the same room as those of zone 1. If zone 2 is contaminated with pathogens, it is likely that zone 1 also becomes contaminated. Contaminant transfer is often caused by human or machine action. Surfaces in zone 2 include walls or floors located near processing equipment and overhanging pipes or equipment. Zone 3 is an area that may cause contamination of zone 2. Zone 3 includes warehouses, employee locker rooms, and loading docks. Listeria monocytogenes is an example of pathogens that are commonly transmitted to food from the processing environment.
Sampled surfaces could be rough or smooth, flat or with curves and corners, continuous or with cracks and crevices, and accessible or difficult to reach. Therefore, the choice of a method to sample a surface depends not only on the surface zone but also on its characteristics.
A sterile cotton swab may be used for limited surfaces or on hard‐to‐reach crevices. The swab is typically made of a wound cotton head (~0.5 cm diameter and 2 cm long) and a 12–15 cm long wooden stick. The swab may be prepared in the laboratory, sterilized, and kept in a sterile container until the time of use. Alternatively, cotton swabs may be purchased as individually wrapped sterile units. In addition to the swab, a sterile rinse solution in a test tube is needed for surface sampling. Many commercially available products include a sterile swab packaged with appropriate diluent in a shatterproof (plastic) tube. A predetermined area (e.g., 100 cm 2) of the surface to be sampled is swabbed with the moistened cotton swab, which is returned to the rinse solution tube. The rinse solution may be diluted serially, and selected dilutions are spread on the surface of a suitable agar growth medium. The inoculated plates are incubated, and populations of the targeted microorganisms are counted. In this scenario, when quantitative results are desired, the rinse solution is considered the undiluted analytical sample.
If the surface to be swabbed is large, or if a microorganism of a low incidence rate in processing environment is sought, a sponge may be used instead of a cotton swab. A natural or synthetic sponge with ~5 × 5 cm contact surface that is free from antimicrobial agents is suitable for this purpose. The sponge can be packed in a heat‐resistant bag or wrapped in aluminum foil and sterilized by the analyst, or purchased prepacked and presterilized from a commercial source. During sampling, the sponge is held aseptically, moistened with 10 ml rinse solution, rubbed against the surface to be sampled and returned to a sterile plastic bag. The sample should be transferred to the laboratory under refrigeration and analyzed without delay. If the purpose of sampling is to detect pathogens, the sponge is transferred to a suitable enrichment broth and the mixture is incubated. When sampling is carried out to quantify environment microbiota, the sponge is mixed with 50‐ or 100‐ml diluent and further dilutions are made. Selected dilutions are then spread on the surface of a suitable agar growth medium, plates are incubated, and the population of the targeted microorganism is counted.
Replicate organism direct agar contact (RODAC) method
The replicate organism direct agar contact (RODAC) method may be used on easily accessible flat surfaces. In this method, Petri plates are filled with an agar medium suitable for the microbiota to be analyzed. These plates may be prepared in the laboratory or purchased from a commercial source. The RODAC plates should contain enough agar medium so that the surface of the medium is convex and rises above the rim of the plate. At the sampling site, the agar medium in the RODAC plate is exposed to the surface being sampled. This exposure is accomplished by pressing the plate against the sampled surface and rolling the plate while applying some pressure. The cover is replaced, and the plate is incubated at a temperature and for a time appropriate to the targeted microorganism or microbiota. After incubation, the colonies on each plate are counted and colony subculturing may follow. Since no sample dilution takes place, the RODAC method is suitable for sampling pre‐cleaned or sanitized surfaces. If the surface is contaminated heavily, incubated RODAC plates will be crowded with colonies and results will be difficult to interpret.
Microorganisms may become airborne due to activities such as water spraying, dry ingredient handling, and vigorous air movements. Air‐suspended dust particles can carry microorganisms. Mold and bacterial spores are common contaminants of air since they survive dryness and other detrimental environmental factors. The microbiological quality of air in a processing facility impacts the quality and safety of perishable food processed in this facility. Improper filtration of air entering a facility or recycling air from the raw product area into the finished product area can result in food contamination. Air quality in the packaging area is particularly important for the control of post‐processing contamination. Therefore, determining the microbial load in air is an important task.
Sedimentation is a simple method to measure air quality. It involves exposing agar media plates to air by leaving these plates uncovered in the location to be sampled. Air contaminants will sediment by the force of gravity during the exposure time (e.g., 15 min). The plates are incubated, and the colony count may be considered proportional to air contamination level.
Air in a particular environment may also be forcibly impacted onto the surface of agar media plates using mechanical means. Jets of air are directed over the media plates so that air load collides and sticks to agar surface. After receiving a measured air sample, the agar plates are incubated, and colonies are counted. Air streams also may be filtered through a microfilter. Microorganisms are released from the filter using a suitable diluent and the microbial load is counted.
Читать дальше













