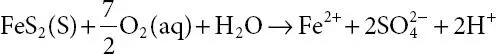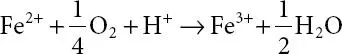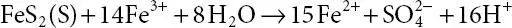Figure 1.1 Schematic showing the pathway of AMD formation, its dispersion into the environment and entrance into the food chain: The destruction of natural vegetation in search of mineral resources exposes large surface areas to weathering effects. Due to the presence of sulfide materials in these mine tailings, AMD products are formed which can be washed away into nearby streams. Aquatic life suffers the consequences due to an increase in mortality rate; meanwhile, these polluted waters can be used for irrigation and thus will bioaccumulate in plants. Once in the food chain, human lives are affected in the process.
1.2 Rare-Earth Element Occurrence in Acid Mine Drainage
1.2.1 Acid Mine Drainage Generation and Effects
Although the formation of AMD has been historically attributed to mine tailing dams containing sulfide-bearing materials, it can occur naturally in an environment that exposes hefty volumes of sulfide-bearing materials to air and water [16]. However, of the different sulfide ore deposits shown in Table 1.1, pyrite is the most common and by far the most abundant sulfide mineral [17]. Using pyrite as an example for the generation of AMD, the oxidation process is represented by different reactions (1–3) [18]. Pyrite is oxidized to sulphate ions  ferrous iron (Fe 2+) and protons (H +) at the initial stage when the tailing dam is exposed to atmospheric oxygen in the presence of excess water at neutral pH ( 1.1).
ferrous iron (Fe 2+) and protons (H +) at the initial stage when the tailing dam is exposed to atmospheric oxygen in the presence of excess water at neutral pH ( 1.1).
(1.1) 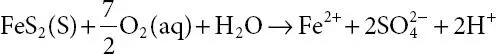
The formation of Fe 2+is solubilized by the oxidation process and subsequently oxidized to ferric iron (Fe 3+) and is the rate-determining step of the overall reaction ( 1.2).
(1.2) 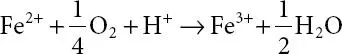
Ferric cations produced can also oxidize additional pyrite into ferrous ions, and the net effect of these reactions is to produce H +, which increases the acidity of the influent and maintains the solubility of the ferric iron ( 1.3).
(1.3) 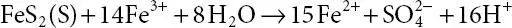
The pH value of AMD is as low as 2–4 and will naturally enhance the rate of dissolution of potentially toxic elements (PTEs), resulting in the tailing dam containing a high content of metal(loids), including sulfate ions in solution. Once leached into the lotic system (river), it will destroy their bicarbonate buffering system and enhance the rate of dissolution of metal ions, which could persist for hundreds of years once initiated, forming an age-long pollution stream with low pH [5, 19]. Consequently, the design lifespan of civil infrastructures such as water reticulation networks and bridges within this environment are shortened, caused by corrosion when metal oxides are formed [20]. In addition, the mortality rate of the aquatic organism within this inhospitable environment increases once these toxic metals accumulate in their organs [21]. More so, when this polluted water enters the irrigational system, the toxic metals accumulate in plants and indirectly enter the food chain. The growth of plants is distorted because, at low pH, plant nutrients such as nitrogen, phosphorus, and potassium necessary for their growth are immobilized, and the calcium and magnesium content becomes deficient. Meanwhile, in the food chain, these toxic metals bioaccumulate and cause deleterious health effects in humans ( Table 1.2) due to their non-biodegradability [22, 23].
Table 1.2 Potential toxic elements and their effect in humans.
| Elements |
Recommended levels in surface or groundwater (ppm) |
Health-related issues in humans |
Reference |
| Aluminum (Al) |
2.9 |
Aluminum exposure is a risk factor for the development or acceleration of the onset of Alzheimer’s disease (AD) in humans. |
[33] |
| Arsenic (As) |
0.01 |
Exposure to arsenic causes skin and internal organ cancers, impaired nerve function, kidney and liver damage, or skin lesions. |
[34] |
| Copper (Cu) |
2.0 |
Exposure to excess copper induces oxidative stress, DNA damage and reduced cell proliferation in the human body. |
[35] |
| Iron (Fe) |
1.0–3.0 |
Iron is an essential part of hemoglobin in humans, but its overload causes severe health problems such as liver cancer, diabetes, cirrhosis of the liver, heart diseases and infertility. |
[36] |
| Manganese (Mn) |
0.5 |
Manganese is an essential nutrient to the body, but in excess can also interfere with the normal function of the nervous system to induce motor and cognitive impairments as well as neuropsychiatric symptoms. |
[37] |
| Lead (Pb) |
0.01 |
Exposure to lead causes cardiotoxicity, neurotoxicity, nephrotoxicity, carcinogenicity and genotoxicity in humans. |
[38] |
| Zinc (Zn) |
5.0 |
Zinc is considered an essential mineral in humans as it is necessary to produce hundreds of enzymes throughout the body. The toxicity of Zn in humans differs significantly and varies from acute exposure to chronic exposure. Renal injury, ranging from asymptomatic hematuria to interstitial nephritis or acute tubular necrosis, has also been reported due to acute toxicity, while chronic exposure can lead to sideroblastic anemia and granulocytopenia, and myelodysplastic syndrome. |
[39] |
| Rare earth elements (REEs) |
Data not established and are currently unregulated in humans and environment |
Despite their extensive application in electronics, exposure to these metals will cause dysfunctional neurological disorders such as reduced intelligence quotient (IQ) in children, bone alteration, genotoxicity and fibrotic tissue injury and antitesticular effects and male sterility in humans. |
[40] |
Table 1.3 Different types of applications and the uses of REEs.
| Types of applications |
REEs used and their functions |
Reference |
| 1. Medical (La, Ce, Pr, Gd, Nd, Tb, Dy, Ho, Er, Tm, and Yb) |
Lanthanum oxide nanoparticles can be used for magnetic resonance imaging (MRI).Cerium-doped lutetium orthosilicate is used to convert high-energy radiation to visible light for positron emission tomography (PET) imaging. This test is used to reveal tissue and organ function. Praseodymium oxide nanoparticles have been synthesized and used for cancer treatment as a radiotherapy technique.The treatment of skin cancer, as well as hair removal using a laser beam, was achieved by using Neodymium as crystals.The magnetic properties of Gadolinium are used to enhance MRI images of tumors and intravenous radio-contrast agents in MRI scans.A radioisotope Dy-165 has been employed in the treatment of rheumatoid knee effusions.Holmium-based solid-state lasers have been used for non-invasive medical procedures for treating cancers and kidney stones.Erbium-based lasers have been used in medical and dental practice.A radioisotope Tm-167 has been used as a power source in portable X-ray devices.A radioisotope Yb-176 can be used to produce Lu-177, which is known to be a promising radioisotope for medical applications. |
[41] |
| 2. Telecommunication |
Neodymium, terbium, and dysprosium are used in smart cell phones to enable them to vibrate. |
[42] |
| 3. Electronics |
Scandium (Sc) is used in electron-beam tubes in TV.Yttrium (Y) is used in the manufacture of capacitors, phosphors, microwave filters, glasses, oxygen sensors, radars, lasers and superconductors.Eu, Tb, Gd, and Ce are used in flat-screen displays. |
[43] |
| 4. Automobile |
La, Ce, Nd, and Pr are used as catalytic converters to efficiently control pollution in cars. |
[44] |
| 5. Weaponry |
Yttrium(Y) and Terbium (Tb) are used for laser targeting and weapons in combat vehicles. |
[45] |
1.2.2 Rare-Earth Elements and Their Importance
Читать дальше

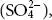 ferrous iron (Fe 2+) and protons (H +) at the initial stage when the tailing dam is exposed to atmospheric oxygen in the presence of excess water at neutral pH ( 1.1).
ferrous iron (Fe 2+) and protons (H +) at the initial stage when the tailing dam is exposed to atmospheric oxygen in the presence of excess water at neutral pH ( 1.1).