Application of Nanotechnology in Mining Processes
Здесь есть возможность читать онлайн «Application of Nanotechnology in Mining Processes» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Application of Nanotechnology in Mining Processes
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Application of Nanotechnology in Mining Processes: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Application of Nanotechnology in Mining Processes»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Nanotechnology has revolutionized processes in many industries but its application in the mining industry has not been widely discussed. This unique book provides an overview of the successful implementation of nanotechnology in some of the key environmental and beneficiation mining processes.
Audience
Application of Nanotechnology in Mining Processes — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Application of Nanotechnology in Mining Processes», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
1 Water Pollution Monitoring and Remediation Initiatives Research Group, School of Chemical and Minerals Engineering, North-West University, Potchefstroom, South Africa
2 Solid-State Research Group, Department of Chemistry, School of Science and Technology, Cape Breton University, Sydney, Canada
3 Faculty of Engineering and the Built Environment, Chemical Engineering Department, Cape Peninsula University of Technology, Cape Town, South Africa
4 Institute for Nanotechnology and Water Sustainability (iNanoWS), College of Science Engineering and Technology (CSET), University of South Africa, Florida Science Campus, Johannesburg, South Africa
5 Department of Electrical and Mining Engineering, College of Science Engineering and Technology (CSET), University of South Africa, Florida Science Campus, Johannesburg, South Africa
Abstract
Mining supplies key resources necessary for technological advancement to ameliorate challenges imposed by the increase in the human population worldwide. One of the legacies of mining resources is the formation and discharge of acid mine drainage (AMD) during and even after active mining. It is a major environmental concern because it enhances the dissolution and increases the dispersion of contaminants, mostly toxic metals, in the environment. Many countries have now adopted or promulgated legislation that requires mining operators to treat and manage the formation of AMD, costing them a fortune from their profits. AMD can be an alternative source of valuable rare earth elements (REE), but the currently available extraction methods of REE from AMD are inefficient and costly, exceeding by many folds their conventional extraction from ores. Thus, there has been a growing effort to develop a novel and cost-effective method to recover REEs from AMD, in which extraction using polymeric nanomaterials, like Poly(amidoamine) (PAMAM) dendrimers, are growing in prominence. PAMAM dendrimers nanoparticles have high adsorption capacity, contributing highly to metal recovery from most wastewater. However, their application in the recovery of REEs from AMD is hampered by the low pH of the AMD, which protonates the amine functional groups forming cationic charges on the surfaces of the dendrimer nanoparticles. Therefore, designing these materials to adsorb metal ions in an acidic solution is paramount for treating AMD. This chapter discusses designing a cost-effective method for the recovery of REEs from AMD after alkaline treatment, using surface-functionalized magnetic PAMAM dendrimer nanoparticles. The environmental effect and shortcomings of AMD remediation methods will be highlighted as a background motivation in developing this procedure.
Keywords: Acid rock drainage, dendrimers, magnetic iron oxides nanoparticle, potentially toxic elements, rare earth element
1.1 Introduction
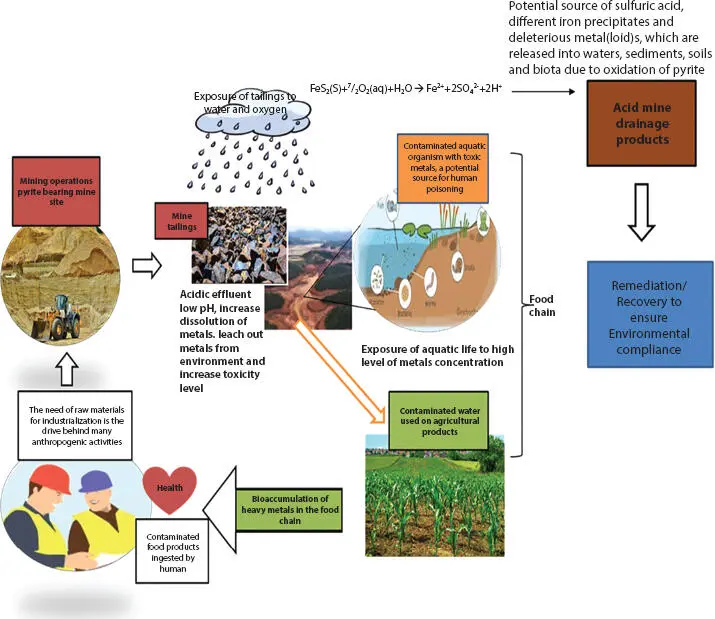
The global human population has risen considerably since the industrial revolution and currently stands at above 7.7 billion worldwide, beyond the carrying capacity of the earth [1]. The rapid population growth is imposing tremendous challenges such as the easy spread of disease outbreaks, food scarcity, shortage of infrastructures, and insufficient communication networks, which require resources and technological advancement to ameliorate these predicaments. All resources required for this technological advancement come from mother earth and can be obtained only through two means; if they cannot be harvested (from farming), they should be mined. The mining industry plays a vital role in supplying these key resources but lacks the potential to obtain mineral resources without compromising environmental integrity [2]. Nevertheless, mining cannot be easily halted due to the growing need for mineral resources to support technological advancement required to artificially sustain the ever-increasing human population, which is beyond the earth’s carrying capacity. The mineral resources, including metals, are essential components in the advancement of several technologies, like the production of medications and vaccines, fertilizers for agricultural application to ensure food security, and the manufacture of building materials for construction of mega-infrastructures to improve road networks. They are also used as vital components to manufacture computers and cell phones for better communication networks. In addition, mining is a vital economic activity for many nations, bringing in much-needed foreign exchange earnings and employment [3]. Despite the benefits mentioned above, the legacy of mining activities includes major environmental pollution and heaps or piles of municipal solid waste (MSW) from mining [4]. For example, most valuable minerals like gold (Au), copper (Cu), sulfur (S), zinc (Zn), silver (Ag) or lead (Pb) occurs in sulfidic ore bodies ( Table 1.1) with more than one type of mineralization [5]. Once these valuable metals have been extracted from their sulfidic ores, vast volumes of mine water and leftover mining solid wastes and tailings are generated, which contain most of the sulfide mineral, like pyrite. The exposure of the pyrite-containing waste to oxygen and water leads to an acid-generating material, producing acid mine drainage (AMD). The discharge of AMD is attributed to most of the contamination being transported from mining sites to the receiving environments, affecting environmental water quality [6–8] ( Figure 1.1). Due to its well-known and publicized ecological impacts, many countries have adopted stringent regulations, such as Section 402 of the Clean Water Act of the Republic of South Africa, enabling mining operators to treat mine water discharging AMD [9–11].
On the one hand, treatment of AMD as required by environmental legislation has serious financial implications for the mining operators, but on the other hand, generation of AMD in former mining sites occurs long after active mining when the responsible mining companies no longer exist. Thus, treatment and remediation of AMD are an economic and financial burden to the mining company if the operation is still active, and to the community and their governments in closed or abandoned mines. Mine water discharging AMD is known to contain a consortium of dissolved elements, including precious metals and rare-earth elements (REE) [12]. Thus, AMD can be a valuable resource, especially of REE and precious metals that can generate more income and compensate for the treatment and remediation expenses of mine water and contaminated mining sites [13]. Furthermore, AMD can be regarded as an initial and natural process of hydrometallurgy of REE. However, extraction of REE from AMD is a big challenge due to the need for highly selective extraction methods, targeting only REE and leaving other metal ions dissolved in AMD to produce the required purity. Thus, recovery of REE from AMD is usually more expensive, resulting in AMD not being attractive as a source of REE. Nevertheless, using polymeric nanomaterials as hydrometallurgical extraction agents is promising to be a cost-effective and efficient method of extracting REE from AMD. One of the agent groups with high potential is Poly(amidoamine) (PAMAM) dendrimers. Consequently, this chapter discusses the application of PAMAM dendrimers as an extraction agent of REE, evaluating their performance potential and the development of methods in which they are applied.
Table 1.1 Different types of sulfide-bearing ore bodies, % content in ore body, uses, world deposit and origin [14, 15].
| Sulfide ore bodies and formula | Minerals present in the ore body with % content | Application of the major elements in the ore body | Regions of major deposit of ore bodies in the world | Origin of occurrence |
| Arsenopyrite (FeAsS) | Arsenopyrite is the major source of arsenic, with 46% Arsenic, 34.3% Iron, and 19.7% Sulfur in the ore body. It should be noted that the arsenic content is made up of minor gold deposits. | Used in the manufacture of herbicide, alloys, wood preservative, medicine, insecticide, and rat poison. | China, Morocco, Namibia, Russia, Belgium, Iran, and Japan | Hydrothermal veins, pegmatite, contact metamorphism, and metasomatism |
| Bornite (Cu5FeS4) | Source of rich-grade copper metal. The ore body contains 63.3% Copper (Cu), 11.1% Iron (Fe), and 25.6% Sulfur (S) | Major applications are in electrical wires, cables, plumbing, currency, utensils, machinery, alloys, architecture, nutritional supplements, and agricultural fungicides | United States, England, Austria, Zimbabwe, Morocco, Dzhezkazgan, and Kazakhstan | In the zone of secondary supergene enrichment, source of rich copper metal |
| Chalcopyrite (CuFeS2) | Chalcopyrite is the principal source of copper metal and accounts for approximately 70% of the copper deposits in the world. The ore body content is made up of 34.5% Copper (Cu), 30.5% Iron (Fe), and 35.0% Sulphur (S) | Major applications of copper metal are in electrical wires, cables, plumbing, currency, utensils, machinery, alloys, architecture, nutritional supplements, agricultural fungicides, and space exploration capsules | Chile, China, Peru, United States, DRC, Australia, Russia, Zambia, Canada, and Mexico | Large, massive, irregular veins, disseminated and porphyry deposit at granitic/dioritic intrusive and SEDEX type |
| Cinnabar ( HgS) | The ore body is a primary source of mercury. It contains 86.2% Mercury (Hg) and 13.8% Sulfur (S). | Used in the manufacturing of industrial chemicals and in electronics, thermometers, medicine, cosmetics, pigments, and fluorescent lamps. Environmentally sensitive due to health and safety regulations | China, Mexico, Kyrgyzstan, Peru, and Tajikistan | Vein-filling by recent volcanic activity and acid-alkaline hot spring |
| Galena (PbS) | Galena is the primary source of lead metal and one of the sources of sulfur. It contains 86.6% Lead (Pb) and 13.4% Sulfur (S). | Used as a key ingredient for paint production, used in plumbing materials, bullets, automobile batteries, alloys, sheets, radiation shields, electrodes, ceramic glazes, stained glass, and cosmetics | China, Australia, United States, Peru, Russia, Mexico, and India | Individually or associated with zinc and copper sulfide deposit |
| Molybdenite (MoS2) | The primary source of molybdenum with 60% Molybdenum (Mo) content and 40% Sulfur (S) | Used to coat stainless steel materials because it is resistant to corrosion, used as lubricant, tools, and high-speed steels, cast iron, electrodes, fertilizers, and for pollution control in power plants | Armenia, Canada, Chile, China, Iran, Mexico, Mongolia, Peru, Russia, and United States | High-temperature hydrothermal ore of chalcopyrite, pyrite, and molybdenite |
| Pentlandite (Fe, Ni)9S8 | The primary source of nickel with 22% Nickel (Ni), 42% Iron (Fe), and 36% Sulfur (S) | Used in stainless steel, superalloys, electroplating, alnico magnets, coinage, rechargeable batteries, electric guitar strings, 4.6 microphone capsules, and green tinted glass | Australia, Namibia, Canada, and Brazil | The layered maficultramafic intrusion at high magmatic temperature, differential segregation |
| Pyrite (FeS2) | Common in all rocks and as massive sulfide deposits. It contains 46.6% Iron (Fe) and 53.4% Sulfur (S) | Mainly used to produce sulfur dioxide for paper and sulfuric acid for the chemical industry. Rarely mined for iron content due to complex metallurgy and being uneconomical commercially. Acid drainage and dust explosion are common hazards with pyrite deposits. | Italy, Spain, Kazakhstan, and United States | Common in all rocks, and as massive sulfide deposits associated with gold |
| Sphalerite (ZnS) | The primary source of zinc with 67% Zinc content and 33% Sulfur (S) | The main applications are in galvanizing, alloys, cosmetics, pharmaceuticals, 3.9 micronutrients for humans, animals, and plants | China, Peru, Australia, United States, Mexico, India, Bolivia, Kazakhstan, Canada, and Sweden | The majority as large SEDEX-type deposits associated with galena, chalcopyrite, and silver |
Интервал:
Закладка:
Похожие книги на «Application of Nanotechnology in Mining Processes»
Представляем Вашему вниманию похожие книги на «Application of Nanotechnology in Mining Processes» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Application of Nanotechnology in Mining Processes» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












