John O'Brien - Earth Materials
Здесь есть возможность читать онлайн «John O'Brien - Earth Materials» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Earth Materials
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Earth Materials: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Earth Materials»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Earth Materials,
Earth Materials,
Earth Materials — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Earth Materials», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Table 4.13 Important rock‐forming mineral polymorphs.
| Chemical composition | Common polymorphs |
|---|---|
| Calcium carbonate (CaCO 3) | Calcite and aragonite |
| Carbon (C) | Diamond and graphite |
| Silica (SiO 2) | α‐quartz, β‐quartz, tridymite, cristobalite, coesite, stishovite |
| Aluminum silicate (AlAlOSiO 4) | Andalusite, kyanite, sillimanite |
| Potassium aluminum silicate (KAlSi 3O 8) | Orthoclase, microcline, sanidine |
| Iron sulfide (FeS 2) | Pyrite, marcasite |
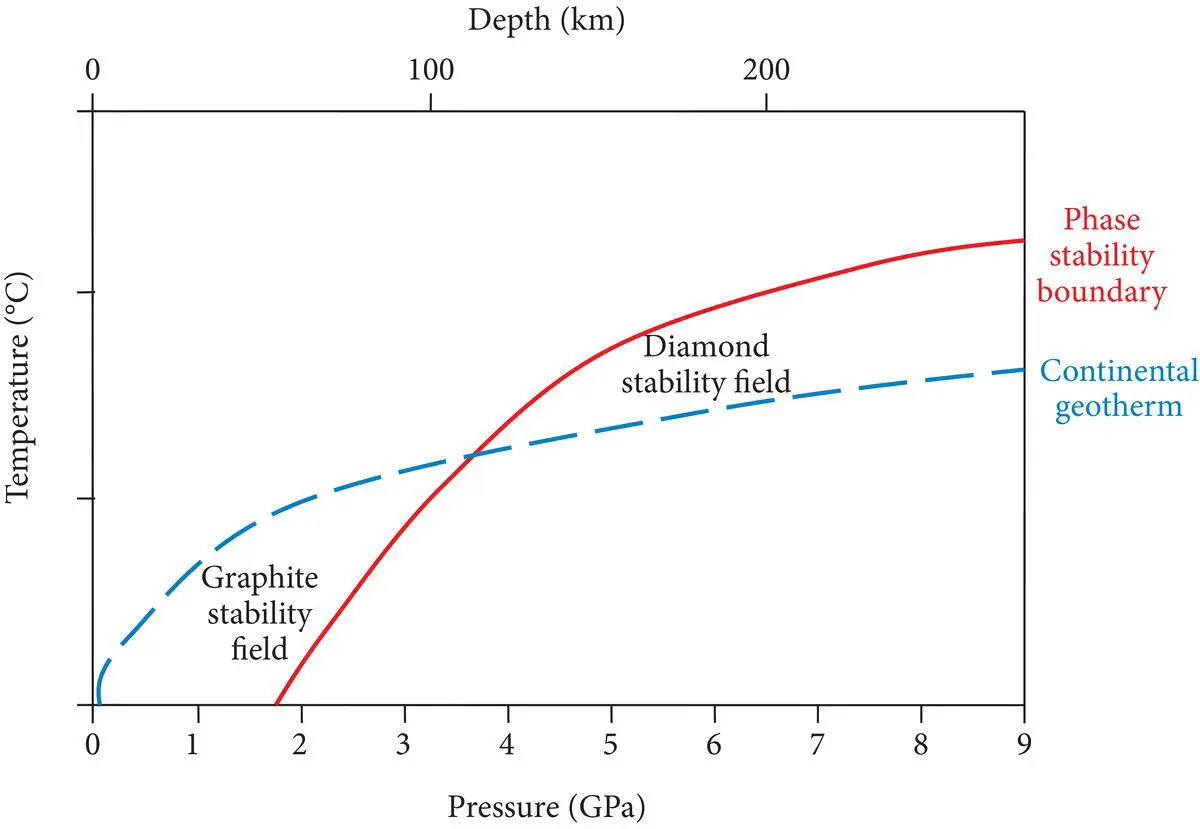
Figure 4.36 Phase stability diagram showing the conditions under which graphite, the low pressure polymorph of carbon, and diamond, the high pressure polymorph of carbon, are stable beneath continental lithosphere.
Reconstructive transformations
Reconstructive transformationsinvolve the conversion of one polymorph (or mineral) into another by processes that require bond breakage so that a significant change in structure occurs. Such transformations require large amounts of energy, and this requirement tends to slow or inhibit their occurrence. In the transformation of diamond to graphite, a large amount of energy is required to break the strong bonds that hold carbon atoms together in the isometric diamond structure, so that they can rearrange into the more open, hexagonal structure of graphite. This inhibits the transformation of diamonds into graphite as diamonds find themselves in lower pressure and lower temperature environments near Earth's surface. Minerals such as diamond that exist under conditions where they are not stable are said to be metastable. All polymorphs that require reconstructive transformations have the potential to exist outside their normal stability ranges as metastable minerals. This allows them to preserve important information about the conditions under which they, and the rocks in which they occur, were formed and, in the case of diamonds, to grace the necks and fingers of people all over the world.
Displacive transformations
Some polymorphs are characterized by structures that, while different, are similar enough that the conversion of one into the other requires only a rotation of the constituent atoms into slightly different arrangements without breaking any bonds. Transformations between polymorphs that do not require bonds to be broken and involve only small rotations of atoms into the new structural arrangement are called displacive transformationsand tend to occur very rapidly under the conditions predicted by laboratory experiments and thermodynamic calculations. Polymorphs involved in displacive transformations rarely occur as metastable minerals far outside their normal stability ranges and so may preserve less information about the conditions under which they and the rocks in which they occur originally formed.
Alpha quartz (low quartz) is generally stable at lower temperatures than beta quartz (high quartz). Although α‐ and β‐quartz have different structures, the structures are so similar ( Figure 4.37) that the conversion of one to the other is a displacive transformation. It is not at all unusual, especially in volcanic rocks, to see quartz crystals with the external crystal form of β‐quartz but the internal structure of α‐quartz. These quartz crystals are interpreted to have crystallized at the elevated temperatures at which β‐quartz is stable and to have been diplacively transformed into the α‐quartz structure as they cooled, while retaining their original external crystal forms.
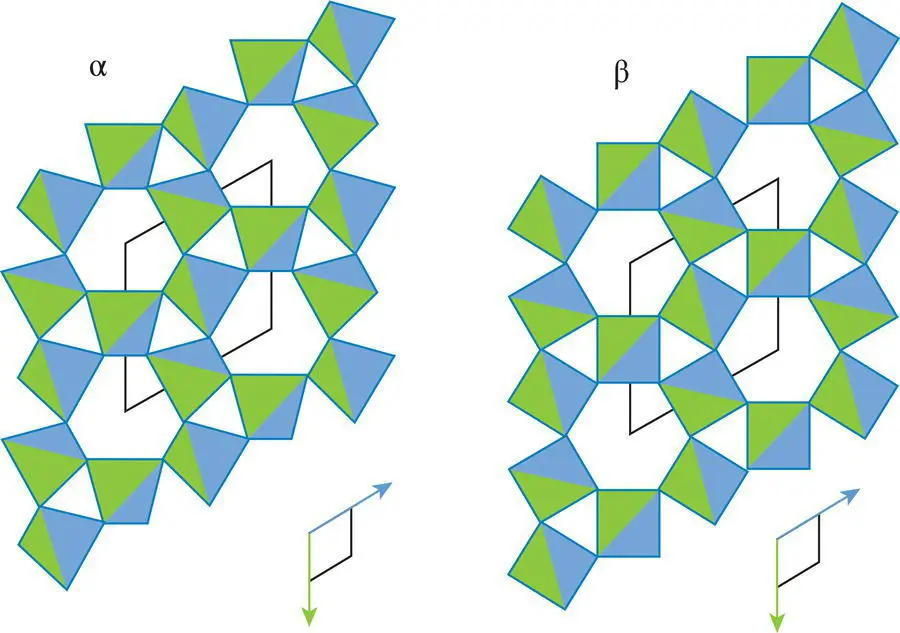
Figure 4.37 The closely similar structures of α‐ and β‐quartz.
Source : Courtesy of Bill Hames.
Other transformations between silica polymorphs are reconstructive. For example, the transformations between the high‐pressure minerals stishovite and coesite and between coesite and quartz are reconstructive. Therefore, both stishovite and coesite can be expected to exist as metastable phases at much lower pressures than those under which they are formed. Their preservation in rocks at low pressures allows them to be used to infer high pressure conditions, such as meteorite impacts, long after such conditions have ceased to exist.
Order–disorder transformations
Many polymorphs differ from one another only in terms of the degree of regularity in the distribution of certain ions within their respective crystal structures. Their structures can range from perfectly ordered to a random distribution of ions within structural sites ( Figure 4.38). The potassium feldspar minerals (KAlSi 3O 8) provide many examples of such variation in regularity or order in the distribution of aluminum ions within the structure. In the feldspar structure, one in every four tetrahedral sites is occupied by aluminum (Al +3), whereas the other three are occupied by silicon (Si +4). In the potassium feldspar high sanidine, the distribution of aluminum cations is completely random (high disorder); the probability of finding an aluminum cation in any one of the four sites is equal. Crystal structures with such random distributions of cations are highly disordered and are favored by high temperatures and low pressures of formation. On the other hand, in the potassium feldspar low microcline, the distribution of aluminum cations is highly ordered (low disorder), with the aluminum distributed regularly in every fourth tetrahedral site. The probability of finding an aluminum cation in these sites approaches 100%, and the probability of finding one in the other three sites approaches zero. Crystal structures with such regular distributions of cations possess very low disorder, and their formation is favored by low temperatures and/or high pressures of formation. Intermediate degrees of order exist within the potassium feldspar group. High sanidine, with its high degree of disorder, crystallizes in the monoclinic system, and is common in volcanic rocks formed at high temperatures and low near surface pressures, whereas low microcline, with its low degree of disorder, crystallizes in the triclinic system, and is common in rocks formed at higher pressures, and in some cases lower temperatures, below the surface.
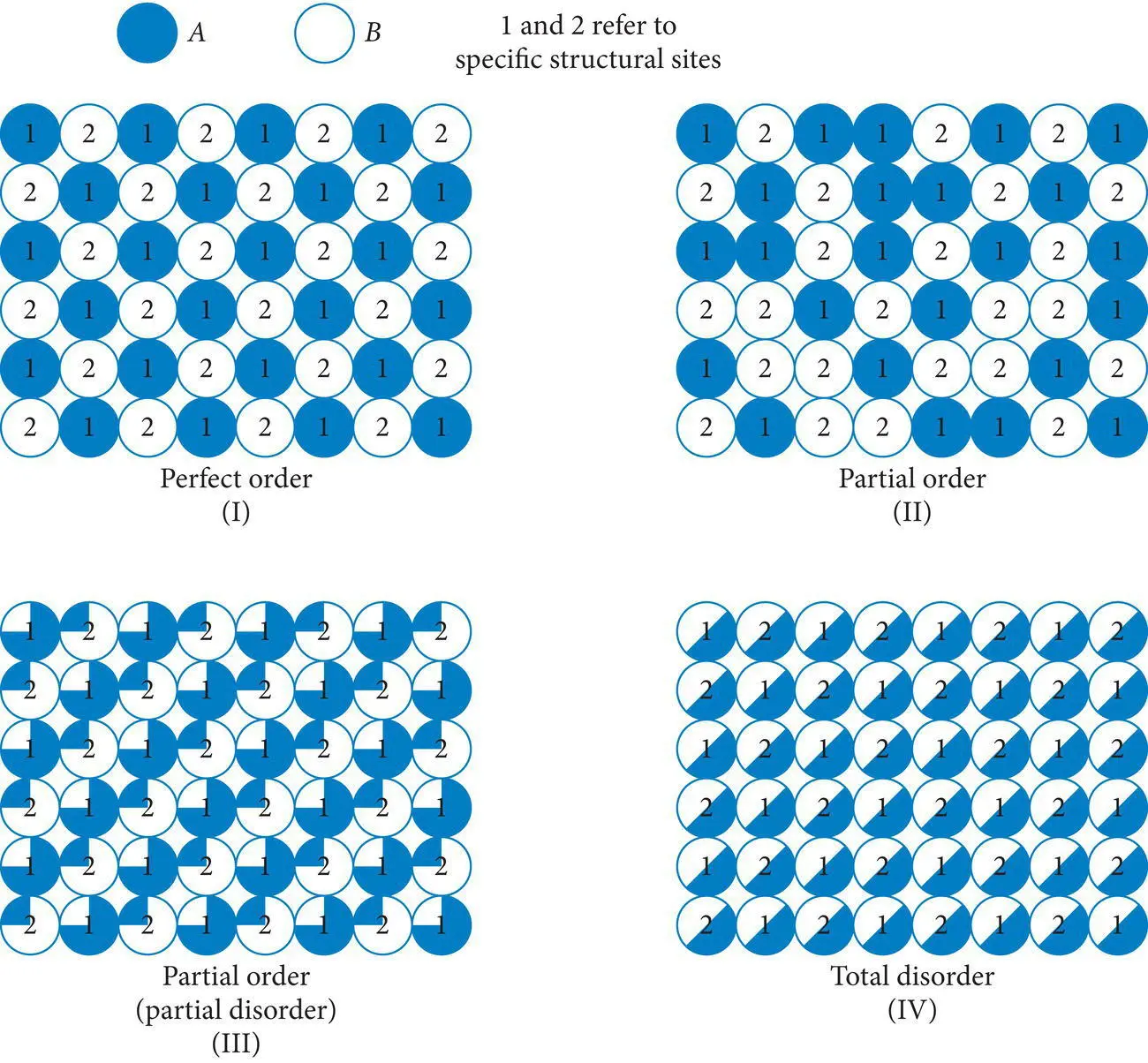
Figure 4.38 Variations in the order of minerals.
Source : Klein and Hurlbut (1985). © John Wiley & Sons.
4.9.2 Pseudomorphs
Minerals that take the crystal form of another, pre‐existing mineral are called pseudomorphs and are said to be pseudomorphic after the earlier mineral ( Figure 4.39). Pseudomorphs can be produced in many ways. All require that the original crystal possessed a significant number of crystal faces (was euhedral or subhedral) at the time it formed. Some pseudomorphs are produced by replacementin which the atoms in a pre‐existing mineral are replaced by the atoms of a new mineral that retains the external crystal form of the original crystal. A common example is the replacement of pyrite (FeS 2) crystals by goethite (FeOOH) to produce goethite pseudomorphs after pyrite. Another common example is quartz (SiO 2) pseudomorphs after fluorite (CaF 2). Some pseudomorphs are castsproduced by dissolution of the old mineral followed by precipitation of the pseudomorph to fill the cavity left behind. Other pseudomorphs are produced by the loss of a constituentfrom the original crystals. For example, the dissolution of carbonate ion from crystals of the copper carbonate mineral azurite [Cu 3(CO 3) 2(OH) 2] can produce native copper (Cu) pseudomorphs after azurite. Still other pseudomorphs are produced when the new mineral forms a thin layer or crust over the original crystal. The encrustationof the original mineral by the new mineral allows the new mineral to mimic the crystal form of the original mineral. Still other pseudomorphs form by inversionas when β quartz crystals are transformed into α quartz, as described in the preceding section.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Earth Materials»
Представляем Вашему вниманию похожие книги на «Earth Materials» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Earth Materials» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












