1 ...6 7 8 10 11 12 ...19 “The three of us could work on that part together,” the professor suggested. “I would like to see what we can come up with.”
The three future “adventurers” finished their tea and cookies with casual conversation about life on campus these days. The students signed up for another office visit at the same time the following week before thanking the professor and taking their leave.
On their way back to the dorm, Marie brought up the subject of pressure as an environmental variable. She wanted to make sure that Helen was comfortable with the idea that pressure, especially static pressure, was a common concept not only in physics and chemistry labs, but also on Earth – where traditional travelers and explorers have gone.
Marie asked Helen, “Did you know that high pressures occur in many places in nature and that they account for many unusual effects? Here on Earth, on the sea floor, there are enormous pressures. Just today in the newspaper I read that at a depth of two kilometers in some sea beds there are hot springs with water coming out at a temperature of 400°C, but there is no bubbling water vapor. That means that the water is not boiling, even at that high temperature! The high pressure ensures that the water remains liquid and is able to carry many minerals with it.
“Pressures are even higher in the deep interior of Earth. The pressures at the center of giant planets and inside the stars are even more extreme. It's hard for us to imagine the effects of these pressures!
“But we don’t have to go to such inaccessible places to the see the effects of high pressure. There are many examples of very high pressure right here on the surface of Earth. Many technological developments such as steam engines, gasoline and diesel engines, missiles and explosives involve very high pressures. High pressure is employed in the industrial processes of the chemical industry such as the manufacture of fertilizers, plastics or even decaffeinated coffee. Recently, high pressure has been used to preserve foods in a better and more efficient manner.
“Perhaps referring to the modern use of high pressure as ‘modern alchemy,’ as Professor Wood tends to do is not such a stretch after all.”
Helen listened quietly and returned a gracious smile to her friend. “Thank you for that,” she said to Marie. “I just need a little time to get myself around all of these different ideas and to make some common sense of them.”
The two friends reached the dorm and went their separate ways. The next week’s classes begged their attention.
The weather the following week had not been conducive to outdoor activities. Professor Wood had made little progress on his sculpture by the time the students appeared at his office door for their next discussion of the world of high pressure - and the possibility of planning some instructive journeys through that strange landscape.
Following the introductory cordialities, Professor Wood opened the conversation. He wanted to address Helen’s concern that she might get “short-changed” from a philosophical point of view.
“Toward the end of our last get-together,” he began, “Helen said some things about atoms and the forces that we now think of as holding atoms together. She also mentioned that the early alchemists only saw the atoms as a-tomos , as indivisible, without any inner structure. But the early alchemists did, in fact, record their thoughts on what they considered to be the basic building blocks of the world as they knew it.
“Today, we think of their ideas more as theoretical speculations. The interaction of different substances was described in more human terms as affinities . You might say that some of the substances displayed a kind of affection for each other. Remember the six-pointed star in the center of the drawing that Marie shared with us? The idea behind that was that all matter ultimately originated from only one kind of matter, the Materia Prima . That is to say, the stuff that Marie described as what there was immediately following the Big Bang.”
“Right! I remember that,” said Helen. She was happy that the conversation had reverted back to historic alchemy.
“In fact,” the professor continued, “the alchemists of the Middle Ages actually were the universal scholars of their time. The alchemists that you found in your reading described the chemical processes of metal refining, medical treatments, ancient Gods, religions and legends, as well as philosophy and natural science in these terms. Solely on the basis of pure logic, for instance, the ancient Greek philosopher Democrituscame to the conclusion that all matter should consists of various small, indivisible particles.
“Since harmony and symmetry played a major role in philosophy at that time, Platoargued that these atoms should have the form of small bodies composed of equilateral triangles. Using multiple triangles, plus some squares and regular pentagons, Plato constructed a number of highly symmetric ‘ideal’ bodies. He regarded these geometric figures to be models for a variety of what he referred to as ‘atoms.’ In Plato’s view, the four forms of matter, Earth, Water, Air and Fire, may be built up by combining different atoms with different shapes. Today, we call these bodies Platonic solids. Take a look at these figures.” The professor picked up another sheet ( Figure 8) from the stack on his desk.
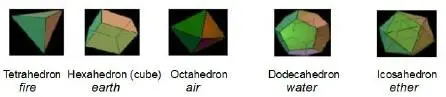
Figure 8. The Platonic Solids5
Helen looked at Plato’s figures. They bore a disturbing similarity to the objects that her high-school math teacher had kept on the top shelf of his book case. Suddenly Helen remembered some models of simple crystals that were on a shelf of the chemistry laboratory. In fact, the professor had some models like those on a shelf next to his desk. ( Figure 9) “It is déjà vu all over again,” Helen thought to herself, paying a brief homage to the contemporary “philosopher” Yogi Berra. Helen began to feel a little uneasy about where this discussion with the professor might be going.

Figure 9. Dense packing of spheres and the rhombic dodecahedron that corresponds to the space around each ball. Any number of these dodecahedra can be combined to form a solid with no space between them.
“Some of these shapes are still relevant to what I mean when I use the term modern alchemy today – but in a somewhat different way,” the professor was saying. He picked up one of the models that Helen had just noticed. It was an assembly of magnetic steel balls that had assumed a solid shape by their attraction to each other.
“Look at how closely the spheres in this model are packed together.” The professor was rotating the model in his hands just as her high-school chemistry teacher had done.
The professor continued his presentation. “If you divide the space around the balls evenly with respect to each of its neighbors, you create a regular body with twelve identical faces. That twelve-sided space is repeated throughout the solid. You can see that, if you then select the same number of balls to represent each edge of the model, the emerging body is called a rhombic dodecahedron. That is because this body has 12 identical rhombic outer surfaces.”
Helen recalled that a rhombus is like a square that has been pushed over at the top. All four sides have equal length, but none of the angles are ninety degrees. The professor referred back to the drawing ( Figure 9) of the model that he was holding. The rhombic dodecahedron appeared on the right-hand side of the picture. Helen’s fears had become reality. She could see in the figure on the right that, even though all of the rhombuses in a three-dimensional solid were identical, they all appeared different in this two-dimensional drawing.
Читать дальше














