“From the little that I know of the atomic theory of matter, the forces inside the nucleus – the ones between the protons and neutrons that largely determine what element the atom represents – are very much larger than the forces that the atoms exert on each other or the forces those atoms are likely to experience in an ordinary college research lab. It’s hard for me to imagine that even modern alchemists can hope to achieve of those kinds of forces and pressures in a college laboratory. Am I right about that, Professor?”
“Well, yes, you are absolutely correct, Helen,” the professor replied. “But I might add that we are able to apply significantly more pressure to materials these days in our college research lab than the alchemists were able to produce using the apparatus that appeared in the drawing of the Alchemist’s Kitchen ( Figure 2) that Marie showed us on her phone. Not only are we able to apply more pressure to the materials, but with a little help from my Philosopher’s Stone here, we are able to extract information about the materials while they are being subjected to high pressure. So even if we are also not able to transmute lead into gold, we may be able to get one element to take on the appearance and some of the other characteristics of another element.”
The professor picked up a strange-looking contraption from his desk. “I’ll explain more about this another time. But to continue the discussion about the relevance of pressure, we notice that at higher pressures the interaction among atoms does change as we apply more and more pressure. If I may go back to the analogy of a globe, I might compare the changes that we have been able to observe in materials to the way the continents change over time due to plate tectonics – or maybe the way politics and war change the shape of countries and the importance of certain cities. Maybe it is some of both. In any case, there are aspects of the way the elements respond to applied pressure that the alchemists would find to be very interesting.”
Marie was anxious to rejoin the conversation. “Are you saying that the changes that some of the elements undergo in the presence of high pressure are such that those materials exhibit different physical properties? Does that mean that, even though one element does not actually become another, it might have the same physical properties under high pressure as has another element? And are you suggesting that your sculpture is somehow able to portray those similarities?”
The professor’s eyes widened at this. Marie’s questions implied a level of perception that was rare among any of the students he had known – far less among first-year college students. He complimented her on her observations, both of which were right on the mark!
“That is very close to what I was hoping that the sculpture would show,” he told her. “That, and a bit more. What I have tried also to capture is some of the significant trends and links that appear within groups of related elements. As I said earlier, the sculpture has much in common with the two-dimensional table of the elements. The way that certain groups of elements behave suggests an underlying principle that is common to that particular group. It’s a little like the way some games have hidden rules that are not visible for a simple spectator of the game. For the initiated player, these rules contribute to the charm of the game. When it comes to the chemical elements, the rules of nature act as lighthouses to help keep us on course.”
“I guess that brings us back to the notion of maps, globes and travel,” Helen interposed. “Is that as far as your travel analogy goes? Your last statement is pretty ambiguous. Even modern day navigators no longer rely upon lighthouses.”
The professor smiled. “There is another analogy that I like to make with regard to travel and global exploration,” he said. “The white spots on the globe today represent only uninhabited fields of ice that are well-defined and charted. In the past, the white areas corresponded to unexplored regions of the globe, but today there is no terra incognita , or unknown land, left anywhere on Earth. You can find satellite images of any place on Earth using Google Earth View! But, on my map there are still other worlds left to explore! The world of high pressure is full of examples. I think that, using different pictures and examples, I might be able to prepare you for a journey through my world of high pressure. You might even want to explore some of the terra incognita we encounter along the way.”
Professor Wood thought that perhaps he had seen Marie stifle a yawn. “Are you sure that you would not like some coffee or tea?” he asked politely.
This time, both students nodded in the affirmative. The professor excused himself to turn on his teapot and set out a mug for each of them. Helen and Marie exchanged glances as the professor placed a mug in front of each of them. The mugs had a peculiar design on them. The design was a “wrap around” of a smaller version of the colorful chart that hung behind the professor’s desk. Both of them had noticed the chart as soon as they had entered the room. It looked a little like the periodic table of elements that had been so much the focus of the conversation for the past half-hour, but the space dedicated to each element contained a blotch of different colors.
A whistle from the teapot signaled the completion of the first step in the preparation of their refreshments. Again, the professor offered the mixed tray of teabags and condiments, plus an attractive plate of sugar cookies. After all, it was getting to be late afternoon!
This time Marie broke the silence as a teabag sank beneath the surface of the water in her decorative mug. “May I infer, Professor, that the white spots on this mug ( Figure 6), and those on the chart behind your desk, ( Figure 7) correspond to the “uncharted territory” that is still left to be explored on the map of your world of high pressure?”

Figure 6. Professor Wood’s Coffee Mugs
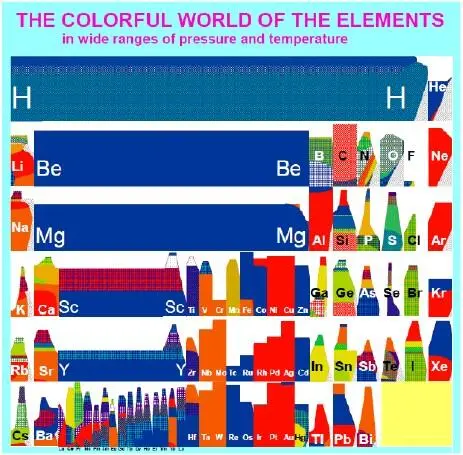
Figure 7. The colorful world of elements under high pressure
“You may, indeed!” Again the professor was amazed at how quickly Marie seemed to be able make connections between disjoint topics of conversation and the artifacts with which he had decorated his walls.
“As you have noted, there are still a lot of places for young researchers to explore! I hope that you like my choice of colors.” The professor continued, “If you were to take a journey with me through the world of high pressure, we would use that chart behind my desk as a world map to keep us oriented.”
“Let us think about that,” Helen said.
“Take your time,” Professor Wood replied. “I’ll be here in any case. I have guided a few other students on such a journey, but they tended to be graduate students or physics majors with advanced standing. From what I have heard from you today, I think that the two of you will get quite a lot out of the experience – especially you, Marie.”
“Of course, I would want Helen to come along,” Marie said glancing at her friend.
Helen seemed a little reticent. “As I said, let me think about it,” she said. “I definitely would want the journey to include more of the philosophy of the ancient and medieval alchemists and the exploratory routes that they followed to get us to where we are today. Oh, and not too much math for me, if you please. I have not had the advanced math courses in Calculus, or whatever it’s called, that Marie has had.”
Читать дальше














