Biomolecules from Natural Sources
Здесь есть возможность читать онлайн «Biomolecules from Natural Sources» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Biomolecules from Natural Sources
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:5 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 100
- 1
- 2
- 3
- 4
- 5
Biomolecules from Natural Sources: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Biomolecules from Natural Sources»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
An up-to-date exploration of new and novel biomolecules Biomolecules from Natural Sources: Advances and Applications,
Biomolecules from Natural Sources: Advances and Applications
Natural Sources: Advances and Applications
Biomolecules from Natural Sources — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Biomolecules from Natural Sources», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Glycolipids could also be theoretically hydrolyzed, as is shown in Figures 1.2 and 1.3. Glycoside hydrolase can hydrolyze the sugar–lipid or sugar–sugar glycosidic bonds (Figure 1.2), while carbohydrate esterase can hydrolyze the sugar–lipid ester bonds (Figure 1.3) (Abdel-Mawgoud and Stephanopoulos 2018).
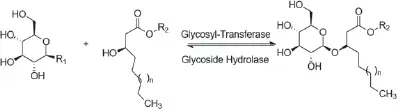
Figure 1.2 Hydrolysis of the sugar–lipid or sugar–sugar glycosidic bonds from glycolipids. R 1: Nucleotides or phosphates groups of the glycosyl residue; R 2: any substitution that could be glycosyl, lipid, or glycolipid units.
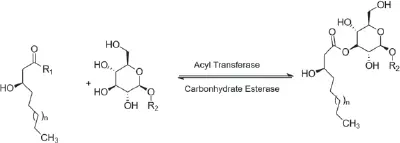
Figure 1.3 Hydrolysis of sugar–lipid ester bonds from glycolipids. R 1: Coenzyme A or Acyl Carrier Protein groups of the acyl donor; R 2: any substitution that could be glycosyl, lipid, or glycolipid units.
1.3 Biosynthesis of Trehalose Lipids
Trehalose lipids are glycolipids containing trehalose hydrophilic moiety. In fact, structurally, trehalose lipids consist of a hydrophilic moiety (trehalose) formed by two glucose units linked through the α,α-1, 1-glycosidic linkage and a hydrophobic moiety represented by chains of fatty acids, such as succinic, octanoic, decanoic, and mycolic acids (Paulino et al. 2016). Trehalose is a non-reducing disaccharide, it is the carbohydrate group of the cell wall glycolipids in Mycobacteria and Corynebacteria (Franzetti et al. 2010). Trehalose displays thermostability, resistance to acid hydrolysis and non-reactivity to the Maillard reaction.
Trehalose lipids were discovered in 1933 (Anderson and Newman 1933) and purified in 1956 (Soberón-Chávez 2011). They are among the best known biosurfactants, as rhamnolipids and sophorolipids, in both composition and activity (Soberón-Chávez 2011). In comparison to other microbial glycolipids, trehalose lipids have shown contrasting results and achievements in both cases of inhibition and enhancement of biodegradation rates (Kügler et al. 2014; Silva et al. 2014). They can reduce the surface tension of aqueous solutions and the interfacial tension between aqueous and oil phases to levels observed with synthetic surfactants, and have low critical micelle concentrations.
A wide variety of trehalose lipids can be found in several forms (Figure 1.4), namely: trehalose monomycolates, dimycolates, trimycolates, non-ionic acylated trehalose derivatives, anionic trehalose tetraesters, and succinoyl trehalolipids (Franzetti et al. 2010; Kügler et al. 2014; Paulino et al. 2016).
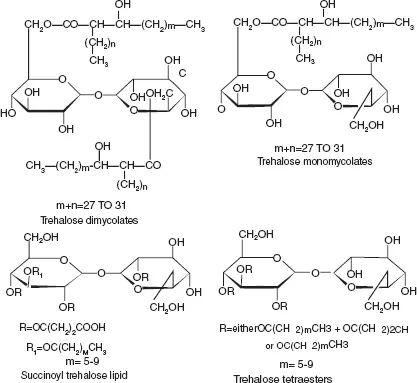
Figure 1.4 The main chemical structure of trehalose lipids (adaped from Franzetti et al. 2010).
Therefore the different types of trehalose lipids can be classified into two general subclasses: The first group is the 6,6-trehalose diesters such as fatty acid trehalose diesters (TDEs, 1), trehalose dicorynomycolates (TDCMs, 2) and trehalose dimycolates (TDMs, 3). The most quantified trehaloselipid is trehalose 6,6´-dimycolate which is an a-branched chain mycolic acid esterified to the C6 position of each glucose (Franzetti et al. 2010). Although TDEs are the simplest glycolipids found in the trehalose 6,6´-diester series, they can be differentiated by lipid length and branching of the fatty acids, which can be divided into anionic or non-anionic. It is known that anionic surface-active TDEs have a higher surface activity than non-ionic (Silva et al. 2014).
The second group is the 2,3-trehalose diesters such as diacyl trehalose sulfates (STL) and sulfolipid-1 (SLL) (Kügler et al. 2014). One of the studies showed that succinoyl trehalose lipids (SCTLs) are promising biosurfactants since they can be efficiently produced from n -alkanes by Rhodococcus bacteria and recovered by precipitation. Among SCTL producers, Rhodococcus sp. SD-74 provided the best yield (40 g L -1) from n -hexadecane under high osmotic conditions. These SCTLs also have also been demonstrated to show growth inhibition against the influenza virus and cell-differentiation induction towards human leukemia cells (Isoda et al. 1997; Sudo et al. 2000).
Mycolic acids are 2-alkyl-3-hydroxy fatty acids of high molecular mass, present exclusively in the cell envelope of bacteria of the mycolata taxa, including Rhodococcus species (Paulino et al. 2016).
The synthesis of mycolic residues, such as trehalose lipids, is believed to be a Claisen condensation. The key reaction for the formation of trehalose-6-phosphate is catalyzed by trehalose-6-phosphate synthetase (TPS). TPS links the two D-glycopyranosyl units at C1 and C1ʹ (Franzetti et al. 2010). Uridine diphosphate-glucose and glucose-6 phosphate participate in that reaction, forming trehalose phosphate (Franzetti et al. 2010):

In alkanotrophic Rhodococci , TPS is induced by n -alkanes. Further reactions for the formation of trehalose lipids have been clearly elucidated for trehalose dimycolates (TDMs) in M. tuberculosis . The mycobacterial glycolipid has been proposed to play a key role in the immunopathogenesis of tuberculosis (Hoq et al. 1997; Jain and Roy 2009; Paulino et al. 2016). The production of threalose lipids established during the final stages of the cell wall synthesis of M.tuberculosis . In this step newly synthesized mycolic acids are transported and attached to the peptidoglycan-arabinogalactan complex of the cell wall, followed by the formation of trehalose dimycolates. This formation occurs in four different reactions (Franzetti et al. 2010, p. 25; Takayama et al. 2005).
The first reaction of the synthesis, is the transfer of the mycolyl group to D-mannopyranosyl-1-phosphoheptaprenol by an as-yet unidentified cytoplasmic mycolyltransferase, forming 6-O-mycolyl-β-D-mannopyranosyl-1-phosphoheptaprenol (Franzetti et al. 2010; Takayama et al. 2005).
The mycolyl group is then transferred to trehalose 6-phosphate by a membrane-associated mycolyltransferase II to form trehalose mono mycolate (TMM)-phosphate. After a dephosphorylation, the resulting product is TMM (Franzetti et al. 2010) (reaction 2) (Figure 1.5).
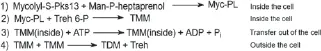
Figure 1.5 Synthesis of trehalose dimycolates, in four different reactions, the first two reactions are performed inside the cell, the third reaction forms the transport out of the cell and the fourth is outside the cell. Pks13: polyketide synthase 13; Man-P-heptaprenol: D-mannopyranosyl-1-phosphoheptaprenol; Myc-PL: 6-O-mycolyl-β-D-mannopyranosyl-1-phosphoheptaprenol; Treh 6-P: trehalose 6-phosphate; TMM: trehalose mono mycolate; TDM: trehalose dimycolate.
The third reaction consists of an extracellular transportation of TMM via an ABC-transporter.
In the fourth reaction trehalose dimycolates(TDM) (Figure 1.5) and arabinogalactan-mycolate are formed from TMM through an extracellular mycolyltransferase (Ag85/Fbp/PS1) (Franzetti et al. 2010; Takayama et al. 2005).
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Biomolecules from Natural Sources»
Представляем Вашему вниманию похожие книги на «Biomolecules from Natural Sources» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Biomolecules from Natural Sources» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.


![Джеймс Купер - Пионеры, или У истоков Саскуиханны [The Pioneers, or The sources of the Susquehannah]](/books/395797/dzhejms-kuper-pionery-ili-u-istokov-saskuihanny-t-thumb.webp)









