Айзек Азимов - The Genetic Effects of Radiation
Здесь есть возможность читать онлайн «Айзек Азимов - The Genetic Effects of Radiation» весь текст электронной книги совершенно бесплатно (целиком полную версию без сокращений). В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Год выпуска: 2018, Издательство: epubBooks Classics, Жанр: Медицина, Биология, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:The Genetic Effects of Radiation
- Автор:
- Издательство:epubBooks Classics
- Жанр:
- Год:2018
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
The Genetic Effects of Radiation: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «The Genetic Effects of Radiation»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The Genetic Effects of Radiation — читать онлайн бесплатно полную книгу (весь текст) целиком
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «The Genetic Effects of Radiation», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Radiation is another matter. In its broadest sense, radiation is any phenomenon spreading out from some source in all directions. Physically, such radiation may consist of waves or of particles. [3] Actually, all waves have some of the characteristics of particles and all particles have some of the characteristics of waves. Usually, however, the radiation is predominantly one or the other and little confusion arises under ordinary circumstances in speaking of waves and particles as though they were separate phenomena.
Of the wave forms the two best–known are sound and electromagnetic radiations.
Sound carries very low concentrations of energy. This energy is absorbed by living tissue and converted into heat. Heat in itself can increase the mutation rate but the effect is a small one. The body has effective machinery for keeping its temperature constant and the gonads are not likely to suffer unduly from exposure to heat.
Electromagnetic radiation comes in a wide range of energies, with visible light (the best–known example of such radiation because we can detect it directly and with great sensitivity) about in the middle of the range. Electromagnetic radiations less energetic than light (such as infrared waves and microwaves) are converted into heat when absorbed by living tissue. The heat thus formed is sufficient to cause atoms and molecules to vibrate more rapidly, but this added vibration is not usually sufficient to pull molecules apart and therefore does not bring about chemical changes.
Light will bring about some chemical changes. It is energetic enough to cause a mixture of hydrogen and chlorine to explode. It will break up silver compounds and produce tiny black grains of metallic silver (the chemical basis of photography). Living tissue, however, is largely unaffected—the retina of the eye being one obvious exception.
Ultraviolet light, which is more energetic than visible light, correspondingly can bring about chemical changes more easily. It will redden the skin, stimulate the production of pigment, and break up certain steroid molecules to form vitamin D. It will even interfere with replication to some extent. At least there is evidence that persistent exposure to sunlight brings about a heightened tendency to skin cancer. Ultraviolet light is not very penetrating, however, and its effects are confined to the skin.
Electromagnetic radiations more energetic than ultraviolet light, such as X rays and gamma rays, carry sufficient concentrations of energy to bring about changes not only in molecules but in the very structure of the atoms making up those molecules.
Atoms consist of particles (electrons), each carrying a negative electric charge and circling a tiny centrally located nucleus, which carries a positive electric charge.
Ordinarily, the negative charges of the electrons just balance the positive charge on the nucleus so that atoms and molecules tend to be electrically neutral. An X ray or gamma ray, crashing into an atom, will, however, jar electrons loose. What is left of the atom will carry a positive electric charge with the charge size proportional to the number of electrons lost.
An atom fragment carrying an electric charge is called an ion . X rays and gamma rays are therefore examples of ionizing radiation .
Radiations may consist of flying particles, too, and if these carry sufficient energy they are also ionizing in character. Examples are cosmic rays , alpha rays , and beta rays . Cosmic rays are streams of positively charged nuclei, predominantly those of the element hydrogen. Alpha rays are streams of positively charged helium nuclei. Beta rays are streams of negatively charged electrons. The individual particles contained in these rays may be referred to as cosmic particles , alpha particles , and beta particles , respectively.
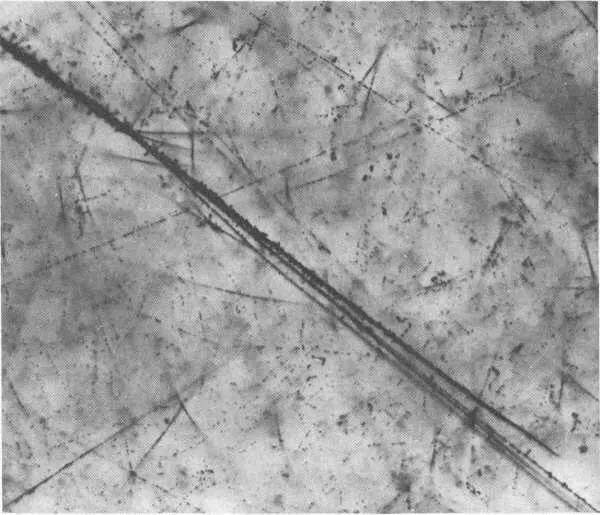
Cosmic ray and trapped Van Allen Belt energetic particles produced the dark tracks in this photo of a nuclear emulsion that had been carried aloft on an Air Force satellite. The energetic particles cause ionization of the silver bromide molecules in the emulsion.
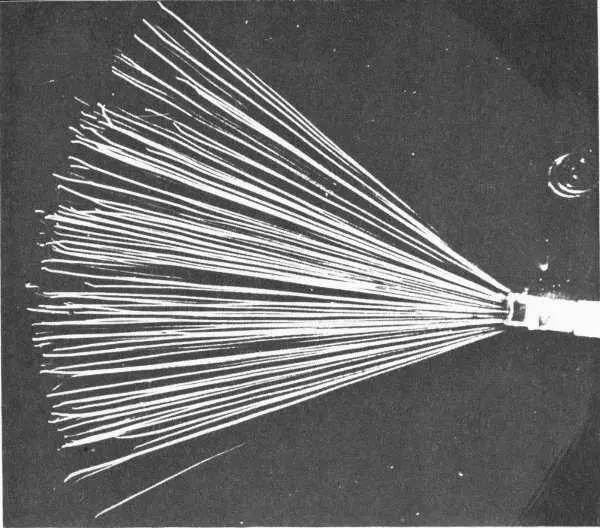
Alpha particles emitted by the source at right leave tracks in a cloud chamber. Some tracks are bent near the end as a result of collisions with atomic nuclei. Such collisions are more likely at the end of a track when the alpha particle has been slowed down.
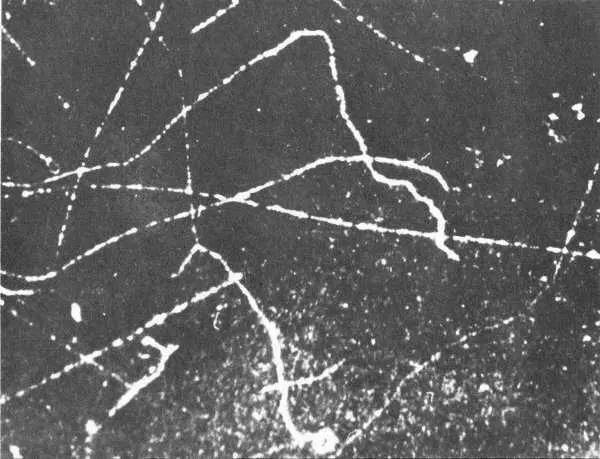
Beta particles originating at left leave these tracks in a cloud chamber. Note that the tracks are much farther apart than those of alpha particles. As the particle slows down, its path becomes more erratic and the ions are formed closer together. At the very end of an electron track the proximity of the ions approximates that in an alpha–particle track.
Ionizing radiation is capable of imparting so much energy to molecules as to cause them to vibrate themselves apart, producing not only ions but also high–energy uncharged molecular fragments called free radicals .
The direct effect of ionizing radiation on chromosomes can be serious. Enough chemical bonds may be disrupted so that a chromosome struck by a high–energy wave or particle may break into fragments. Even if the chromosome manages to remain intact, an individual gene along its length may be badly damaged and a mutation may be produced.
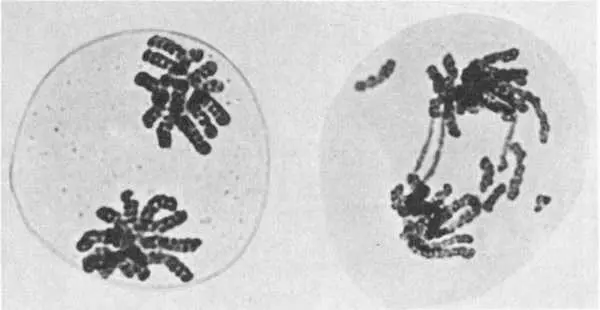
Effects of ionizing radiation on chromosomes: Left, a normal plant cell showing chromosomes divided into two groups; right, the same type of cell after X–ray exposure, showing broken fragments and bridges between groups, typical abnormalities induced by radiation.
If only direct hits mattered, radiation effects would be less dangerous than they are, since such direct hits are comparatively few. However, near–misses may also be deadly. A streaking bit of radiation may strike a water molecule near a gene and may break up the molecule to form a free radical. The free radical will be sufficiently energetic to bring about a chemical reaction with almost any molecule it strikes. If it happens to strike the neighboring gene before it has disposed of that energy, it will produce the mutation as surely as the original radiation might have.
Furthermore, ionizing radiations (particularly of the electromagnetic variety) tend to be penetrating, so that the interior of the body is as exposed as is the surface. The gonads cannot hide from X rays, gamma rays, or cosmic particles.
All these radiations can bring about somatic mutations—all can cause cancer, for instance.
What is worse, all of them increase the rate of genetic mutations so that their presence threatens generations unborn as well as the individuals actually exposed.
Background Radiation
Ionizing radiation in low intensities is part of our natural environment. Such natural radiation is referred to as background radiation . Part of it arises from certain constituents of the soil. Atoms of the heavy metals, uranium and thorium, are constantly, though very slowly, breaking down and in the process giving off alpha rays, beta rays, and gamma rays. These elements, while not among the most common, are very widely spread; minerals containing small quantities of uranium and thorium are to be found nearly everywhere.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «The Genetic Effects of Radiation»
Представляем Вашему вниманию похожие книги на «The Genetic Effects of Radiation» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «The Genetic Effects of Radiation» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.








![Айзек Азимов - Земля Ханаанская. Родина иудаизма и христианства[The Land of Canaan]](/books/172206/ajzek-azimov-zemlya-hanaanskaya-rodina-iudaizma-i-h-thumb.webp)



