Cesium is in the same atomic family as potassium, and it mimics its chemical characteristics. This makes cesium particularly dangerous because it ensures that as a contaminant it will be ingested, since potassium is required by all living things. Cesium is recycled (along with potassium) in soil as a macronutrient, and this process tends to keep cesium in the top soil layers. [6] Plants and animals in both aquatic and terrestrial ecosystems tend to accumulate cesium as they would potassium. This is especially true for terrestrial organisms rich in potassium, such as fungi and berries. Cesium uptake in plants can be somewhat limited by the addition of potassium fertilizers; it is also taken up less in aquatic systems where waters are murkier.
Scientists now believe that it will be 180 to 320 years before the cesium-137 that contaminated much of Belarus, Ukraine, Russia, and Europe actually disappears from the ecosystems. [7] A. Madrigal, “Chernobyl Exclusion Zone Radioactive Longer Than Expected,” Wired.com, December 15, 2009.
INTENSELY RADIOACTIVE FISSION PRODUCTS VERSUS NATURALLY OCCURRING RADIONUCLIDES
Fission products produced by nuclear power plants and weapons, such as cesium-137 and strontium-90, are something new to us as a species. These radionuclides did not exist on Earth in any appreciable quantities during the entire evolution of complex life. Although they are invisible to our senses, they are millions of times more poisonous than most of the common poisons we are familiar with. They cause cancer, leukemia, genetic mutations, birth defects, malformations, and abortions at concentrations almost below human recognition and comprehension. They are lethal at the atomic or molecular level.
These radionuclides emit radiation—invisible forms of matter and energy that we might compare to fire because radiation burns and destroys human tissue. But unlike the fire of fossil fuels, radioactivity cannot be extinguished because it comes from the disintegration of single atoms.
Radioactivity is a term that indicates how many radioactive atoms are disintegrating in a time period. We measure the intensity of radioactivity by the rate of disintegration and the energy this produces. One becquerel equals one atomic disintegration (transformation) per second. One curie, which equals 37 billion becquerels, is defined as the amount of any radioactive material that will decay at a rate of 37 billion disintegrations per second. [8] I realize that these are considered “old” terms to describe radioactivity, but they are the most straightforward and easiest to understand for most people. I think that the numerous changes in terminology in radiological science have in part been done to obscure such meanings from nontechnical audiences.
Sometimes these man-made radionuclides are compared to naturally occurring radionuclides, such as potassium-40, which is found in bananas and other fruits. But this is a false comparison since most naturally occurring long-lived radioactive elements, commonly found in Earth’s crust, are very weakly radioactive. [9] There are highly radioactive naturally occurring radionuclides, such as radon and its daughter product, polonium, which have very short half-lives. These are not commonly found in foodstuffs because they self-destruct long before they can make it into the food chains.
Note that potassium-40 has a specific activity of 71 ten-millionths of a curie per gram. Compare that to 88 curies per gram for cesium-137 and 140 curies per gram for strontium-90. [10] The 88 curies per gram includes the decay process of cesium-137 to barium-137m, in which the barium-137m emits high-powered gamma radiation. Barium-137m has a half-life of not quite three minutes.
In other words, cesium-137 is 12 million times more radioactive than potassium-40. This is like comparing an atomic bomb to a stick of dynamite. Another highly radioactive fission product, strontium-90, releases almost 20 million times more radiation per unit mass than potassium-40. Which one of these would you rather have in your bananas?
Current radiation safety exposure standards use mathematical models to calculate the internal “committed” dose of radiation delivered by any given quantity of ionizing radiation. These models average the dose of ionizing radiation over the mass of the organ system or tissue mass where it occurs. This approach essentially ignores—and thus dismisses—the intensity of the given source and instead focuses upon the total amount of radiation released in the tissue. [11] The models use weighting factors to multiply the estimated biological effects of various atomic particles (twenty times for alpha particles) and the given tissue where it resides. But the multiplication is quickly negated by many orders of magnitude when the dose given to a tiny cluster of cells is averaged over the organ system or body area in which the tiny cluster of cells resides.
In other words, the models equate the effects of a large amount of diffuse, naturally occurring radiation with that from a small, highly concentrated source as long as they both contain the same total amount of energy. If the total energy in a large bucket of warm water is equivalent to that in a tiny, burning piece of coal, does drinking the warm water have the same biological effect as swallowing the coal?
TOXICITY OF CESIUM-137
The amount of cesium-137 deposited per square kilometer (or square mile) of land defines the degree to which an area is classified as being too radioactive to work or live in. To get an idea of the extreme toxicity of cesium-137, consider how little of it is required to make a large area of land uninhabitable for more than a century.
The lands that were grossly contaminated by the destruction of the Chornobyl nuclear power plant are classified by the number of curies of radiation per square kilometer. Strict radiation-dose control measures were imposed in areas contaminated to levels greater than 15 curies per square kilometer of cesium-137. The total area of this radiation-control zone is huge: 10,000 square kilometers, or 3,861 square miles, which is nearly half the area of the state of New Jersey. [12] R. Alvarez, J. Beyea, K. Janberg, J. Kang, E. Lyman, A. Macfarlane, G. Thompson, and F. von Hippel, “Reducing the Hazards from Stored Spent Power-Reactor Fuel in the United States,” Science and Global Security 11 (2003): 7.
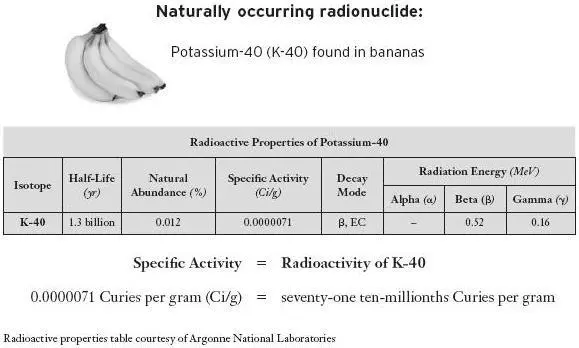
Figure 5.1. Weakly Radioactive Naturally Occurring Radionuclide
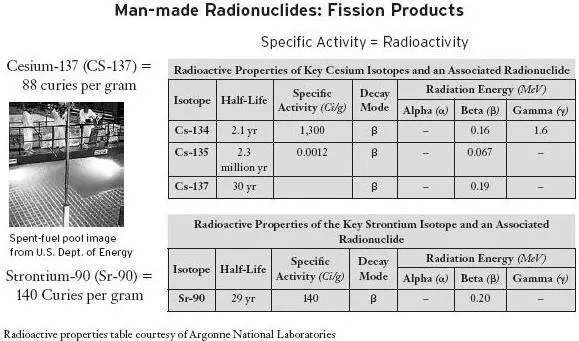
Figure 5.2
The 1,100-square-mile uninhabitable exclusion zone that surrounds the destroyed Chornobyl reactor has greater than 40 curies of radioactivity per square kilometer, or 104 curies per square mile.
Consider again that one gram of cesium-137 has 88 curies of radioactivity. This means that as little as one-third of a gram of cesium-137, evenly distributed as smoke or a gas over an area of one square kilometer, will make that square kilometer into a radioactive exclusion zone. Less than two grams of cesium-137—a quantity less than half the weight of an American dime—if made a radioactive gas or aerosol and evenly distributed over an area of one square mile, will turn that square mile into a radioactive exclusion zone that will remain uninhabitable for one hundred to two hundred years. For example, the 1,317 square miles of Central Park in New York City can be made uninhabitable for more than a century by less than two grams of cesium-137.
Читать дальше














![Helen Rowland - The Widow [To Say Nothing of the Man]](/books/752764/helen-rowland-the-widow-to-say-nothing-of-the-man-thumb.webp)