Children, especially girls, are at the most risk from radiation-induced cancer. In fact, a female infant has a seven times greater risk, and a five-year-old girl has a five times greater risk, of getting a radiation-induced cancer than does a thirty-year-old man. Currently accepted radiation safety standards actually use a “reference man,” who is twenty to thirty years of age, as the basis for the standards, which underestimates the dose for infants and children. [19] A. Makhijani, “The Use of Reference Man in Radiation Protection Standards with Recommendations for Guidance and Change,” Institute for Energy and Environmental Research, December 2008.
There is a great deal of controversy in regards to the accuracy of the methods used to calculate the millisievert, especially the accurate determination of the biological effects of exposure to a source of ionizing radiation that is external to the body versus the long-term internal exposure to the living cells adjacent to radioactive atoms or particles that have been ingested, inhaled, or absorbed by the body.
The risk factors created by the ICRP radiation models were derived largely from studies of Japanese atomic bomb survivors, who received a homogeneous, high-dose, short external exposure to mainly gamma radiation. The ICRP radiation safety models make the basic assumption that these risk factors can be applied to heterogeneous, low-dose, internal exposure to ionizing radiation. Furthermore, the ICRP standards only cite risks for fatal cancer and include an added element for nonfatal cancer and genetic effects. But noncancer effects from radiation, such as cardiovascular effects, are not included. [20] Ibid.
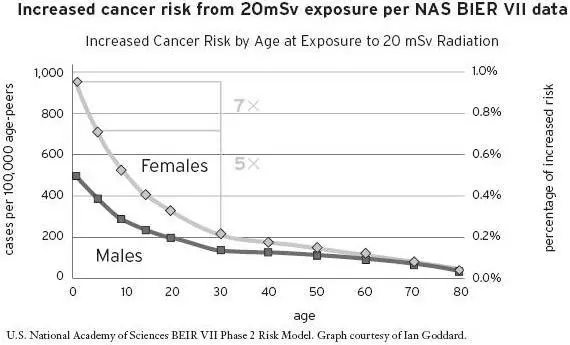
Figure 5.5
The criteria used for determining the evacuation zones was the millisievert (millisievert = one–one thousandth or 0.001 sievert), which is not a measured quantity of radiation, such as the curie or becquerel. Sieverts represent the biological effects of ionizing radiation. A sievert is a derived number, based upon the mathematical models used to convert the measured “absorbed dose” to an “effective,” “equivalent,” or internal “committed effective dose.” [21] These conversions are made through the multiplication of hypothesized “radiation weighting factors” times the absorbed dose in order to obtain the equivalent dose, and through the subsequent multiplication of hypothesized “tissue weighting factors” times the equivalent dose to obtain a total effective dose. “Effective dose coefficients” are also calculated and then used to calculate an internal dose that becomes a “committed” dose, averaged over time, after the radionuclide enters the body.
Even the measured absorbed dose includes an important assumption. The absorbed dose averages the amount of energy deposited over a defined mass or volume of tissue, taking no account of the distribution of the energy within the tissue. In other words, the absorbed dose implies that this averaging process will provide sufficient information for the practical application under consideration. [22] Committee Examining Radiation Risks of Internal Emitters, London, “Report of the Committee Examining Radiation Risks of Internal Emitters (CERRIE),” October 2004.
It is this approach that equates the “dose” from a weakly radioactive isotope such as potassium-40 with that of an intense radionuclide such as cesium-137. Such a simplistic assumption cannot be made when dealing with biological processes at the atomic and molecular levels.
INCREASED INTERNAL EXPOSURE OF CESIUM-137 THROUGH BIOACCUMULATION AND BIOMAGNIFICATION
In the contaminated land surrounding Chornobyl and Fukushima, the primary route of internal exposure is through the ingestion of foodstuffs contaminated with cesium-137, which tends to bioaccumulate in plants and animals. [23] Significant quantities of cesium-137 may also be making their way into human bodies through the inhalation of radioactive smoke. This is because in most rural homes of Belarus and Ukraine, cooking and heating is done with wood, and the wood in contaminated areas has become radioactive. Burning the wood releases its radioactivity. Vassili Nesterenko, a prominent Soviet physicist who lived in Belarus (he died in 2008), stated that chimneys in these households became “miniature nuclear reactors” after constantly burning radioactive wood.
Cesium-137 has a 110-day biological half-life, meaning that half of it will be excreted from the human body 110 days after it is ingested, inhaled, or absorbed. Like other industrial toxins, cesium-137 often cannot be excreted faster than it is being ingested, so it accumulates and increases its concentration in the plant or animal that is routinely ingesting it.
Cesium-137 also tends to biomagnify as it moves up the food chain. This means it becomes progressively more concentrated in predator species. We have seen this before with other industrial toxins, such as DDT, which can magnify its concentration millions of times from the bottom to the top of a food chain.
Consequently, many of the foodstuffs in a contaminated region tend to contain cesium-137. Those naturally rich in potassium, such as mushrooms and berries, can often have very high concentrations of cesium-137. Dairy products and meats, which come from higher levels of the food chain, will also tend to have higher concentrations.
The International Commission on Radiological Protection (ICRP), which sets radiation safety standards, recognizes that cesium-137 bioaccumulates in humans. Figure 5.6, which comes from the ICRP, compares a single ingestion of 1,000 becquerels of cesium-137, a one-time exposure, with the daily ingestion of 10 becquerels. With the single exposure, note that half the cesium-137 is gone from the body in 110 days.
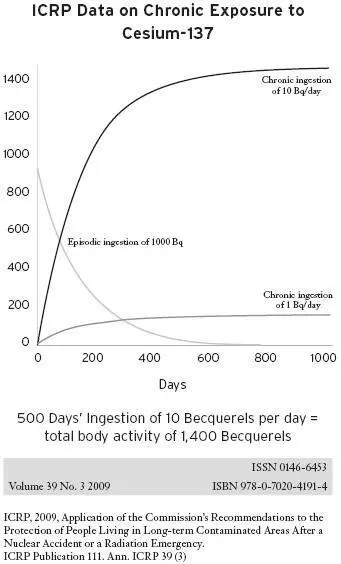
Figure 5.6
With the routine, daily ingestion of 10 becquerels of cesium-137, the total radioactivity within the body continues to rise until after about 500 days, when there is a total of more than 1,400 becquerels of radioactivity measured in the body.
Becquerels can be counted in living persons because the decay of cesium-137 leads to the emission of gamma radiation, which passes through the body and can be measured by a whole-body counter. In a 70-kilogram adult, a total-body activity of 1,400 becquerels would correspond to 20 becquerels per kilogram of body weight; in a 20-kilogram child, this same total would represent 70 becquerels per kilogram of body weight. The ICRP does not specify the average age or weight of those examined in the study, but the safety standards that have been set by the nuclear industry do not consider this level of chronic exposure to so-called low-dose radiation to be a significant danger to human health.
The ICRP states in the document that a whole-body activity of 1,400 becquerels is equivalent to an exposure of 0.1 millisieverts per year. The ICRP radiation models, used by radiation health physicists to convert this level of internal absorbed dose to millisieverts, do not predict serious health risks from such exposures. The models predict that it is safe to have ten times this exposure level. [24] ICRP, “Application of the Commission’s Recommendations to the Protection of People Living in Long-Term Contaminated Areas After a Nuclear Accident or a Radiation Emergency,” Annals of the ICRP 39, no. 3 (2009).
BIOACCUMULATION OF CESIUM-137 IN HUMANS
There is, however, strong evidence that the ingestion of these levels of “low-dose” radiation is, in fact, particularly injurious to infants and children. Research done by Dr. Yuri Bandazhevsky and his colleagues and students in Belarus from 1991 through 1999 correlated whole-body radiation levels of 10 to 30 becquerels per kilogram of whole-body weight with abnormal heart rhythms, and levels of 50 becquerels per kilogram of body weight with irreversible damage to the tissues of the heart and other vital organs. Their findings were first published by a Swiss medical journal in 2003.
Читать дальше














![Helen Rowland - The Widow [To Say Nothing of the Man]](/books/752764/helen-rowland-the-widow-to-say-nothing-of-the-man-thumb.webp)