William Abney - Colour Measurement and Mixture
Здесь есть возможность читать онлайн «William Abney - Colour Measurement and Mixture» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: foreign_antique, foreign_prose, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Colour Measurement and Mixture
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Colour Measurement and Mixture: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Colour Measurement and Mixture»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Colour Measurement and Mixture — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Colour Measurement and Mixture», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
The existence of both kinds of these dark rays may be demonstrated in a very simple manner by the effect that they produce on certain bodies. For instance, there is a yellow dye with which cheap ribbon is dyed, which if placed in the spectrum and beyond the violet causes a visible prolongation of the spectrum. The light in the newly-seen and once invisible part of the spectrum is yellow, the colour of the ribbon itself. In fact, the whole of that part of the spectrum, which on the white screen is seen as blue and violet, becomes yellow, the red and green remaining unchanged. This change in colour is due to fluorescence, a phenomenon of light which Sir G. Stokes found was caused by an alteration in the lengths of the waves of light when reflected from certain bodies. It is not meant to imply by this that the wave-length of any ray falling on a body can be altered by reflection, but only that the body itself on which the rays fall emits rays of light which are not of the same wave-length as those which fall upon it. Now it is a fact that the rays that lie beyond the violet, and which are ordinarily invisible, are shorter than the violet rays, and that these are shorter than the yellow rays. It follows therefore that when, what we may now call, the ultra-violet rays fall on the yellow dyed ribbon, the waves emitted by it are so lengthened that they appear yellow to the eye instead of dark, violet, or blue.
We can also brush a solution of quinine on the screen, and immediately the place where the ultra-violet rays fall is illuminated by a violet light. We do not see the ultra-violet rays themselves, but only the rays of increased wave-length, which are emitted by their effect on the sulphate of quinine. Common machine oil as used for engines also emits greenish rays when excited by the ultra-violet rays, and a very beautiful colour it is. Fluorescence then is one means of demonstrating the existence of the ultra-violet rays – or Ritter's rays as they were formerly called, after their discoverer – in a very simple manner. The method of rendering the effects of the infra-red rays visible to the eye is also interesting. All, or at all events most, of our readers have seen Balmain's luminous paint. A glass or card coated with this substance, which is essentially a sulphide of calcium, when exposed to the light of the sun, or of the electric arc, and then taken into comparative darkness, is seen to shine with a peculiar violet-coloured light. If when thus excited we place it in a bright spectrum for some little time, we shall find on shutting off the light that where the ultra-violet and blue fell on it, the violet light is intenser than the light of the main part of the screen; where the yellow fell there is neither increase or diminution in brightness; but that in the red it becomes darker, and also beyond the limit of the visible spectrum, indicating the existence of rays beyond, which through their greater length have not the power of affecting the eye. If the spectrum be shut off, however, very soon after it falls on the plate, it has been asserted that the red and infra-red rays have increased the brightness of that particular part of the plate on which they fell. At first these two observations seem to contradict one another; they do not in reality. We may expose a tablet of Balmain's paint to light, and place a heated iron in contact with the back of the plate; we shall then find that the iron produces a bright image of its surface on a less bright background. This bright image will gradually fade away, and the same space will eventually become dark compared with the rest of the plate. The reason of this is clear. When light excites the paint a certain amount of energy is poured into it, which it radiates out slowly as light. When the hot iron is placed in contact with it, the heat causes the light to radiate more rapidly, and consequently with greater intensity, at the part where its surface touches, and the energy of that particular portion becomes used up. When the energy of radiation of this part becomes less than that of the rest of the tablet, its light must of necessity be of less brightness than that of the background, with which the heated iron has had no contact. For this reason the image of the iron subsequently appears dark. We shall see presently, and as before stated, that the principal heating effect of the spectrum lies in the red and infra-red, and it is owing to the heating of the paint by these rays that the image might be at first slightly brighter than the background, and subsequently darker.
There is another way in which the existence of both the ultra-violet and infra-red rays can be demonstrated, and that is by means of photography. If we place an ordinary photographic plate in the spectrum and develop it, we shall find that besides being affected by the blue and violet rays, it is also affected by the rays beyond the violet, the energy of these rays being capable of causing a decomposition of the sensitive silver salt. If quartz prisms and lenses be used, and the electric light be the source of illumination, the ultra-violet spectrum will extend to an enormous extent. A more difficult, but perhaps even more interesting means of illustrating the existence of the infra-red rays, and first due to the writer, can be made by means of photography. It is possible to prepare a photographic plate with bromide of silver, which is so molecularly arranged that it becomes capable of being decomposed not only by the violet and blue rays, but also by the red rays, and by those rays which have wave-lengths of nearly three times that of the red rays. It would be inappropriate to enter into a description of the method of the preparation of these plates. Those who are curious as to it will find a description in the Bakerian lecture published in the Philosophical Transactions of the Royal Society for 1881. With plates so prepared it has been found possible to obtain impressions in the dark with the rays coming from a black object, heated to only a black heat.
That these dark rays possess greater energy or capacity for doing work of some kind than any other rays of the spectrum, can be shown by means of a linear thermopile (Fig. 4), if it be so arranged as to allow only a narrow vertical slice of light to reach its face.
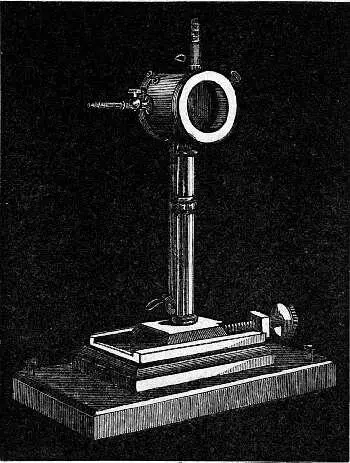
Fig. 4. – The Thermopile.
The principle of the thermopile we need not describe in detail. Suffice it to say that the heating of the soldered junctions of two dissimilar metals (there are ten pairs of antimony and bismuth in the above instrument) produces a feeble current of electricity, which, however, is sufficient to cause a deflection to the suspended needle of a delicate galvanometer. To the needle is attached a mirror weighing a fraction of a grain, and the deflections are made visible by the reflection from it of a beam of light issuing from a fixed point along a scale. The greater the heating of the junctions of the thermopile, within limits which in these cases are never exceeded, the greater is the current produced, and consequently the greater is the deflection of the mirror-bearing needle, and of the beam of light along the scale. In order to get a comparative measure of the energies of the different rays, it is necessary that they should be completely absorbed. Now the junctions themselves of the pile being metal, and therefore more or less bright, will not absorb completely, but if they be coated with a fine layer of lamp-black, the rays falling on the pile will be absorbed by this substance, and their absorption will cause a rise in temperature in it, and the heat will be communicated to the thermopile.
If we make a bright spectrum, and one not too long, say three inches in length, and pass the linear thermopile through its length, we shall find that when the galvanometer is attached, the galvanometer needle will be differently deflected in its various parts. The deflection will be almost insensible in the violet, but sensible in the blue, rather more in the green, still more in the yellow, and it will further increase in the red. When, however, the slit of the thermopile is placed beyond the limit of the visible spectrum, the deflection enormously increases, and will increase till a position is reached as far below the red as the yellow is above it. After this maximum is reached, by moving the pile still further from the red, the galvanometer needle will travel towards its zero, and finally all deflection will cease. At this point we may suppose we have reached the limit of the spectrum, but if rock-salt prisms and lenses be used, the limit will be increased. What the real limit of the spectrum is, is at present unknown; Mr. Langley with his bolometer, and rock-salt prisms, an instrument more sensitive than the thermopile, must have nearly reached it.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Colour Measurement and Mixture»
Представляем Вашему вниманию похожие книги на «Colour Measurement and Mixture» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Colour Measurement and Mixture» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












