Robert DeCourcy Ward - Practical Exercises in Elementary Meteorology
Здесь есть возможность читать онлайн «Robert DeCourcy Ward - Practical Exercises in Elementary Meteorology» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: foreign_antique, Физика, foreign_edu, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Practical Exercises in Elementary Meteorology
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Practical Exercises in Elementary Meteorology: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Practical Exercises in Elementary Meteorology»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Practical Exercises in Elementary Meteorology — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Practical Exercises in Elementary Meteorology», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Galileo (1564-1642) first taught that the air has weight. His pupil Torricelli went a step further. Torricelli saw that the column of water, held up by the pressure of the air in the tube of the pump, must exactly balance a similar column of air, reaching from the surface of the water in the well to the top of the atmosphere. The column of water, in other words, exactly replaces this column of air. While working on this subject, Torricelli, in 1643, performed the following experiment. He filled a glass tube, about 3 feet long and closed at one end, with mercury. After filling the tube, he put his finger over the open end and inverted the tube over a vessel containing mercury. When the lower end of the tube was below the surface of the mercury in the dish, he removed his finger. At once the column of mercury fell in the tube until it stood at a height of about 30 inches, leaving a vacant space of 6 inches in the upper part of the tube. This space has since been known as the Torricellian vacuum . Torricelli had proved what he had expected, viz., that the height of the column of liquid which replaces and balances an air column of the same size varies with the weight of that liquid. It takes a column of water 34 feet long to balance a similar column of air. It takes a column of mercury only 30 inches long to balance a similar column of air. This, as Torricelli correctly explained, is due to the fact that mercury is so much (13 1⁄ 2times) heavier than water. The column of water weighs just the same as the column of mercury. Each column exactly balances an air column of similar cross-section. The height of the water or of the mercury is a measure of the weight or pressure of the air. The greater the pressure on the surface of the water in the well, the higher will be the top of the water in the pump. The greater the pressure on the surface of the mercury in the basin, in the experiment of Torricelli, the higher will the mercury column stand in the glass tube. Either water or mercury may be used as the liquid in the barometer. Otto von Guericke (1602-1686), of Magdeburg, constructed a water barometer about 36 feet long, which he attached to the outside wall of his house. This barometer he used for some months, and made predictions of coming weather changes by means of it. A water barometer is, however, a very unwieldy thing to manage, on account of the great length of its tube. Furthermore, water barometers cannot be used in any countries where the temperatures fall to freezing. Mercury is the liquid universally employed in barometers. It is so heavy that only a small column of it is necessary to balance the atmospheric pressure. Therefore a mercurial barometer is portable. Further, mercury does not freeze until the temperature falls to 40° below zero.
Another name which should be mentioned in connection with the barometer is that of Blaise Pascal, who in 1648 fully confirmed Torricelli’s results. Pascal saw that if the mercury column is really supported by the weight of the air, the height of that column must be less on the summit of a mountain than at the base, because there is less air over the top of the mountain than at the bottom, and therefore the weight of the air must be less at the summit. To prove this, he asked his brother-in-law Perrier, who lived at Clermont, in France, to carry the Torricellian tube up the Puy-de-Dôme, a mountain somewhat over 3500 feet high in Central France. This Perrier did on Sept. 19, 1648, and he found, as predicted by Pascal, that the mercury fell steadily in the tube as he went up the mountain, and that at the top of the mountain the column of mercury was over 3 inches shorter than at the base.
The pressure of the atmosphere is a weather element which, unlike the other elements already considered, cannot be observed without an instrument. We cannot, under ordinary conditions at sea level, determine by any of our senses whether the pressure is rising or falling, or is stationary. The pressure on the upper floors of one of our high buildings is shown by a barometer to be considerably lower than it is at the level of the street below, and yet we notice no difference in our feelings at the two levels.
It is only when we ascend far into the air, as in climbing a high mountain or in a balloon, that the much-diminished pressure at these great heights perceptibly influences the human body. Mountain climbers and aëronauts who reach altitudes of 15,000 to 20,000 feet or more, usually suffer from headache, nausea, and faintness, which have their cause in the reduced pressure encountered at these heights.

Fig. 5.
The ordinary mercurial barometer in use to-day is, essentially, nothing more than the glass tube and vessel of Torricelli’s famous experiment. A simple form of the mercurial barometer is shown in Fig. 5. It consists of a glass tube about one-quarter of an inch in inside diameter and about 36 inches long. This tube, closed at the top and open at the bottom, is filled with mercury, the lower, open end dipping into a cup of mercury known as the cistern . The space above the mercury is a vacuum. The mercury extends inside the tube to a height corresponding to the weight or pressure of the air, the vertical height of the top of the mercury column above the level of the mercury in the cistern, in inches and hundredths of an inch, being the barometer reading. At sea level the normal barometer reading is about 30 inches. There is an opening near the top of the cistern, at the back of the instrument, through which the air gains access to the mercury and holds up the mercury column. It will readily be seen that, as the mercury in the tube rises, the level of the mercury in the cistern falls, and vice versa , so that there is a varying relation between the two levels. In order to have the reading accurate, it is necessary that the surface of the mercury in the cistern should be just at the zero of the barometer scale when a reading is made. To accomplish this, the bottom of the cistern consists of a buckskin bag which may be raised or lowered by means of a thumb-screw, seen at the lower end of the instrument. The level of the mercury may thus be changed and adjusted to the top of a black line, marked on the outside of the cistern, and which indicates the zero of the scale. Before making a reading, the surface of the mercury in the cistern must be raised or lowered until it just reaches this black line. Then the top of the mercury column will give the pressure of the air. The reading is made on an aluminum scale at the top of the wooden back on which the tube is mounted, this scale being graduated both on the English and on the metric system. This barometer may be hung against the wall of a room.
The aneroid barometer(Greek: without fluid ), although less desirable in many ways than the mercurial, is nevertheless a useful instrument for rough observations. The aneroid is not good for careful scientific work, because its readings are apt to be rather inaccurate. To be of much value in indicating exact pressures, it should frequently be compared with and adjusted to a mercurial barometer. An ordinary aneroid barometer is shown in Fig. 6.
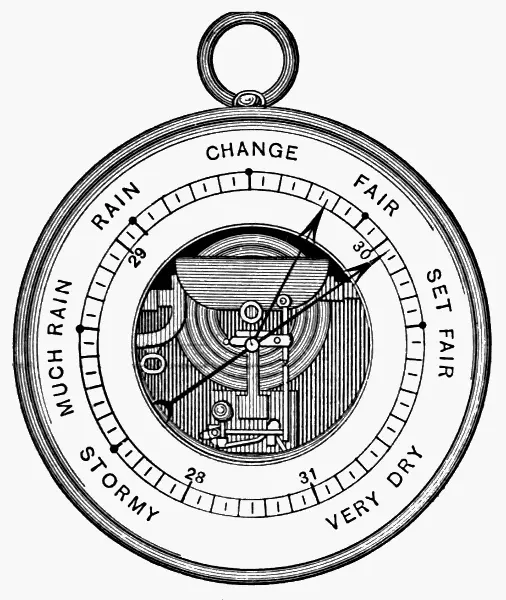
Fig. 6.
In this instrument the changes in atmospheric pressure are measured by their effects in altering the shape of a small metallic box, known as the vacuum chamber . The upper and lower surfaces of this box are made of thin circular sheets of corrugated German silver, soldered together around their outer edges, thus forming a short cylinder. From this the air is exhausted, and it is then hermetically sealed. A strong steel spring, inside or outside of the vacuum chamber, holds apart the corrugated surfaces, which tend to collapse, owing to the pressure of the external air upon them. An increase or decrease in the air pressure is accompanied by an approach, or a drawing apart, of the surfaces of the chamber. These slight movements are magnified by means of levers, a chain, and a spindle, and are made to turn an index hand or pointer on the face of the instrument. The outer margin of the face, underneath the glass, is graduated into inches and hundredths, and the pressure may thus be read at once.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Practical Exercises in Elementary Meteorology»
Представляем Вашему вниманию похожие книги на «Practical Exercises in Elementary Meteorology» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Practical Exercises in Elementary Meteorology» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












