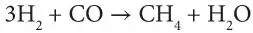Several important chemical reactions, and a host of side reactions, are involved in the manufacture of low heat-content gas under the high-temperature conditions employed (2011; Speight, 2013). Low heat-content gas contains several components, four of which are always major components present at levels of at least several percent; a fifth component, methane, is marginally a major component.
The nitrogen content of low heat-content gas ranges from somewhat less than 33% v/v to slightly more than 50% v/v and cannot be removed by any reasonable means; the presence of nitrogen at these levels makes the product gas low heat-content by definition. The nitrogen also strongly limits the applicability of the gas to chemical synthesis. Two other noncombustible components (water, H 2O, and carbon dioxide, CO) further lower the heating value of the gas; water can be removed by condensation and carbon dioxide by relatively straightforward chemical means.
The two major combustible components are hydrogen and carbon monoxide; the H 2/ CO ratio varies from approximately 2:3 to approximately 3:2. Methane may also make an appreciable contribution to the heat content of the gas. Of the minor components hydrogen sulfide is the most significant and the amount produced is, in fact, proportional to the sulfur content of the feed coal. Any hydrogen sulfide present must be removed by one, or more, of several procedures (Mokhatab et al. , 2006; Speight, 2019).
Producer gas is a low Btu gas typically obtained from a coal gasifier (fixed-bed) upon introduction of air instead of oxygen into the fuel bed. The composition of the producer gas is approximately 28% v/v carbon monoxide, 55% v/v nitrogen, 12% v/v hydrogen, and 5% v/v methane with some carbon dioxide. Water gas is a medium Btu gas which is produced by the introduction of steam into the hot fuel bed of the gasifier. The composition of the gas is approximately 50% v/v hydrogen and 40% v/v carbon monoxide with small amounts of nitrogen and carbon dioxide. Also, low heat-content gas is of interest to industry as a fuel gas or even, on occasion, as a raw material from which ammonia, methanol, and other compounds may be synthesized.
Medium Btu gas (medium heat-content gas) has a heating value in the range 300 to 550 Btu/ ft 3) and the composition is much like that of low heat-content gas, except that there is virtually no nitrogen. The primary combustible gases in medium heat-content gas are hydrogen and carbon monoxide (Kasem, 1979). Medium heat-content gas is considerably more versatile than low heat-content gas; like low heat-content gas, medium heat-content gas may be used directly as a fuel to raise steam, or used through a combined power cycle to drive a gas turbine, with the hot exhaust gases employed to raise steam, but medium heat-content gas is especially amenable to synthesize methane (by methanation), higher hydrocarbon derivatives (by Fischer-Tropsch synthesis), methanol, and a variety of synthetic chemicals.
The reactions used to produce medium heat-content gas are the same as those employed for low heat-content gas synthesis, the major difference being the application of a nitrogen barrier (such as the use of pure oxygen) to keep diluent nitrogen out of the system.
In medium heat-content gas, the H 2/CO ratio varies from 2:3 C to 3:1 and the increased heating value correlates with higher methane and hydrogen contents as well as with lower carbon dioxide contents. Furthermore, the very nature of the gasification process used to produce the medium heat-content gas has a marked effect upon the ease of subsequent processing. For example, the CO 2-acceptor product is quite amenable to use for methane production because it has (i) the desired H 2/CO ratio just exceeding 3:1, (ii) an initially high methane content, and (iii) relatively low water and carbon dioxide contents. Other gases may require appreciable shift reaction and removal of large quantities of water and carbon dioxide prior to methanation.
Town gas is also a medium Btu that is produced in the coke ovens and has the approximate composition: 55% v/v hydrogen, 27% v/v methane, 6% v/v carbon monoxide, 10% v/v nitrogen, and 2% v/v carbon dioxide. Carbon monoxide can be removed from the gas by catalytic treatment with steam to produce carbon dioxide and hydrogen.
High Btu gas (heat-content gas) is essentially pure methane and often referred to as synthetic natural gas or substitute natural gas (SNG) (Kasem, 1979; c.f. Speight, 1990, 2013). However, to qualify as substitute natural gas, a product must contain at least 95% methane, giving an energy content (heat content) of synthetic natural gas on the order of 980 to 1080 Btu/ft 3).
The commonly accepted approach to the synthesis of high heat-content gas is the catalytic reaction of hydrogen and carbon monoxide:
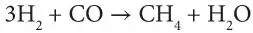
To avoid catalyst poisoning, the feed gases for this reaction must be quite pure and, therefore, impurities in the product are rare. The large quantities of water produced are removed by condensation and recirculated as very pure water through the gasification system. The hydrogen is usually present in slight excess to ensure that the toxic carbon monoxide is reacted; this small quantity of hydrogen will lower the heat content to a small degree.
The carbon monoxide/hydrogen reaction is somewhat inefficient as a means of producing methane because the reaction liberates large quantities of heat. In addition, the methanation catalyst is troublesome and prone to poisoning by sulfur compounds and the decomposition of metals can destroy the catalyst. Hydrogasification may be thus employed to minimize the need for methanation:

The product of hydrogasification is far from pure methane and additional methanation is required after hydrogen sulfide and other impurities are removed.
Synthetic natural gas (SNG) is methane obtained from the reaction of carbon monoxide or carbon with hydrogen. Depending on the methane concentration, the heating value can be in the range of high-Btu gases.
Synthesis gas is a mixture mainly of hydrogen and carbon monoxide which is comparable in its combustion efficiency to natural gas (Speight, 2008 Chapter 7). This reduces the emissions of sulfur, nitrogen oxides, and mercury, resulting in a much cleaner fuel (Nordstrand et al. , 2008; Lee et al. , 2006; Sondreal et al. , 2004, 2006; Yang et al. , 2007; Wang et al. , 2008). The resulting hydrogen gas can be used for electricity generation or as a transport fuel. The gasification process also facilitates capture of carbon dioxide emissions from the combustion effluent (see discussion of carbon capture and storage below).
Although synthesis gas can be used as a stand-alone fuel, the energy density of synthesis gas is approximately half that of natural gas and is therefore mostly suited for the production of transportation fuels and other chemical products. Synthesis gas is mainly used as an intermediary building block for the final production of various fuels such as synthetic natural gas, methanol, and synthetic fuel (dimethyl ether – synthesized gasoline and diesel fuel) (Chadeesingh, 2011; Speight, 2013).
The use of synthesis gas offers the opportunity to furnish a broad range of environmentally clean fuels and chemicals and there has been steady growth in the traditional uses of synthesis gas. Almost all hydrogen gas is manufactured from synthesis gas and there has been an increase in the demand for this basic chemical. In fact, the major use of synthesis gas is in the manufacture of hydrogen for a growing number of purposes, especially in crude oil refineries (Speight, 2014a, 2017). Methanol not only remains the second-largest consumer of synthesis gas but has shown remarkable growth as part of the methyl ethers used as octane enhancers in automotive fuels.
Читать дальше