Both metals and non‐metals exist in nature in the native form, where essentially only one element exists in the structure. Metals occurring in the native form include copper, silver, gold, and platinum which are all characterized by cubic close packing of atoms, high densities, and are malleable and soft. The carbon atoms in diamond are linked in tetrahedral groups forming well cleaved, very hard, translucent crystals. Sulfur also occurs as rings of eight atoms and forms bipyramids or is amorphous.
1 Gold – Au
2 Silver – Ag
3 Platinum – Pt
4 Palladium – Pd
5 Copper – Cu
1 Sulfur – S
2 Diamond – C
3 Graphite – C
The halide mineral group comprises compounds made up by ionic bonding. Minerals such as halite and sylvite are cubic, have simple chemical formulae, and are highly soluble in water. Halides sometimes form as ore minerals, such as chlorargyrite and atacamite.
1 Halite – NaCl
2 Sylvite – KCl
3 Chlorargyrite – AgCl
4 Fluorite – CaF2
5 Atacamite – Cu2Cl(OH)3
This is a large group of minerals in which bonding is both ionic and covalent in character. The sulfide group has the general formula A MX P, where X is typically S but can be As, Sb, Te, Bi, or Se, and A is one or more of the metals. The sulfosalts, which are less common than sulfides, have the general formula A MB NX P, where A is usually Ag, Cu, or Pb, B is commonly As, Sb, or Bi, and X is S. The sulfide and sulfosalt minerals are generally opaque, dense, and have a metallic to sub‐metallic luster.
1 Chalcocite – Cu2S
2 Bornite – Cu5FeS4
3 Galena – PbS
4 Sphalerite – ZnS
5 Chalcopyrite – CuFeS2
6 Pyrrhotite – Fe1–xS
7 Pentlandite – (Fe,Ni)9S8
8 Millerite – NiS
9 Covellite – CuS
10 Cinnabar – HgS
11 Skutterudite – (Co,Ni)As3
12 Sperrylite – PtAs2
13 Braggite/cooperite – (Pt,Pd,Ni)S
14 Moncheite – (Pt,Pd)(Te,Bi)2
15 Laurite – RuS2
16 Cobaltite – CoAsS
17 Gersdorffite – NiAsS
18 Loellingite – FeAs2
19 Arsenopyrite – FeAsS
20 Molybdenite – MoS2
21 Realgar – AsS
22 Orpiment – As2S3
23 Stibnite – Sb2S3
24 Bismuthinite – Bi2S3
25 Argentite – Ag2S
26 Calaverite – AuTe2
27 Pyrite – FeS2
1 Tetrahedrite – (Cu,Ag)12Sb4S13
2 Tennantite – (Cu,Ag)12As4S13
3 Enargite – Cu3AsS4
This group of minerals is variable in its properties but is characterized by one or more metals in combination with oxygen or a hydroxyl group. The oxides and hydroxides typically exhibit ionic bonding. The oxide minerals can be hard, dense, and refractory in nature (magnetite, cassiterite) but can also be softer and less dense, forming as products of hydrothermal alteration and weathering (hematite, anatase, pyrolusite). Hydroxides, such as goethite and gibbsite, are typically the products of extreme weathering and alteration.
1 Cuprite – Cu2O
2 Hematite – Fe2O3
3 Ilmenite – FeTiO3
4 Hercynite – FeAl2O4
5 Gahnite – ZnAl2O4
6 Magnetite – Fe3O4
7 Chromite – FeCr2O4
8 Rutile – TiO2
9 Anatase – TiO2
10 Pyrolusite – MnO2
11 Cassiterite – SnO2
12 Uraninite – UO2
13 Thorianite – ThO2
14 Columbite‐tantalite – (Fe,Mn)(Nb,Ta)2O6
Hydroxides (or Oxyhydroxides)
1 Goethite – FeO(OH)
2 Gibbsite – Al(OH)3
3 Boehmite – AlO(OH)
4 Manganite – MnO(OH)
The carbonate group of minerals form when anionic carbonate groups 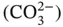 are linked by intermediate cations such as Ca, Mg, and Fe. Hydroxyl bearing and hydrated carbonates can also form, usually as a result of weathering and alteration. The other oxysalts, such as the tungstates, sulfates, phosphates, and vanadates, are analogous to the carbonates, but are built around an anionic group in the form
are linked by intermediate cations such as Ca, Mg, and Fe. Hydroxyl bearing and hydrated carbonates can also form, usually as a result of weathering and alteration. The other oxysalts, such as the tungstates, sulfates, phosphates, and vanadates, are analogous to the carbonates, but are built around an anionic group in the form 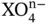 .
.
1 Calcite – CaCO3
2 Dolomite – CaMg(CO3)2
3 Ankerite – CaFe(CO3)2
4 Siderite – FeCO3
5 Rhodochrosite – MnCO3
6 Smithsonite – ZnCO3
7 Cerussite – PbCO3
8 Azurite – Cu3(OH)2(CO3)2
9 Malachite – Cu2(OH)2CO3
1 Scheelite – CaWO4
2 Wolframite – (Fe,Mn)WO4
1 Baryte(s) – BaSO4
2 Anhydrite – CaSO4
3 Alunite – KAl3(OH)6(SO4)2
4 Gypsum – CaSO4·2H2O
5 Epsomite – MgSO4·7H2O
1 Xenotime – YPO4
2 Monazite – (Ce,La,Th)PO4
3 Apatite – Ca5(PO4)3(F,Cl,OH)
1 Carnotite – K2(UO2)(VO4)2·3H2O
The bulk of the Earth's crust and mantle is made up of silicate minerals that can be subdivided into several mineral series based on the structure and coordination of the tetrahedral 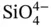 anionic group. Silicate minerals are generally hard, refractory, and translucent. Most of them cannot be regarded as ore minerals in that they do not represent the extractable part of an ore body, and the list provided below shows only some of the silicates more commonly associated with mineral occurrences as gangue or alteration products. Some silicate minerals, such as zircon and spodumene, are ore minerals and represent important sources of metals such as zirconium and lithium, respectively. Others, such as kaolinite, are mined for their intrinsic properties (i.e. as a clay for the ceramics industry).
anionic group. Silicate minerals are generally hard, refractory, and translucent. Most of them cannot be regarded as ore minerals in that they do not represent the extractable part of an ore body, and the list provided below shows only some of the silicates more commonly associated with mineral occurrences as gangue or alteration products. Some silicate minerals, such as zircon and spodumene, are ore minerals and represent important sources of metals such as zirconium and lithium, respectively. Others, such as kaolinite, are mined for their intrinsic properties (i.e. as a clay for the ceramics industry).
1 Quartz – SiO2
2 Orthoclase – (K,Na)AlSi3O8
3 Albite – (Na,Ca)AlSi3O8
4 Scapolite – (Na,Ca)4(Al,Si)4O8)3 (Cl, CO3)
5 Zeolite (analcime) – NaAlSi2O6·H2O
1 Zircon – Zr(SiO4)
2 Garnet (almandine) – Fe3Al2(SiO4)3
3 Garnet (grossular) – Ca3Al2(SiO4)3
4 Sillimanite – Al2SiO5
5 Topaz – Al2SiO4(F,OH)2
6 Chloritoid – (Fe,Mg,Mn)2(Al,Fe)Al3O2(SiO4)2(OH)4
1 Beryl – Be3Al2Si6O18
2 Tourmaline – (Na,Ca)(Mg,Fe,Mn,Al)3(Al,Mg,Fe)6Si6O18(BO3)3(OH,F)4
1 Lawsonite – CaAl2Si2O7(OH)2·H2O
2 Epidote – Ca2(Al,Fe)3Si3O12(OH)
1 Kaolinite – Al4Si4O10(OH)8
2 Montmorillonite – (Na,Ca)0.3(Al,Mg)2Si4O10(OH)2·nH2O
3 Illite – KAl2(Si,Al)4O10(H2O)(OH)2
4 Pyrophyllite – Al2Si4O10(OH)2
5 Talc – Mg3Si4O10(OH)2
6 Muscovite – KAl2(AlSi3O10)(OH)2
7 Biotite – K(Fe,Mg)3(Al,Fe)Si3O10(OH,F)2
8 Lepidolite – K(Li,Al)3(Si,Al)4O10(OH,F)2
9 Chlorite – (Fe,Mg,Al)5–6(Si,Al)4O10(OH)8
1 Tremolite‐actinolite – Ca2(Fe,Mg)5Si8O22(OH)2
2 Spodumene – LiAlSi2O6
3 Wollastonite – CaSiO3
Читать дальше

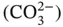 are linked by intermediate cations such as Ca, Mg, and Fe. Hydroxyl bearing and hydrated carbonates can also form, usually as a result of weathering and alteration. The other oxysalts, such as the tungstates, sulfates, phosphates, and vanadates, are analogous to the carbonates, but are built around an anionic group in the form
are linked by intermediate cations such as Ca, Mg, and Fe. Hydroxyl bearing and hydrated carbonates can also form, usually as a result of weathering and alteration. The other oxysalts, such as the tungstates, sulfates, phosphates, and vanadates, are analogous to the carbonates, but are built around an anionic group in the form 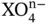 .
.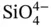 anionic group. Silicate minerals are generally hard, refractory, and translucent. Most of them cannot be regarded as ore minerals in that they do not represent the extractable part of an ore body, and the list provided below shows only some of the silicates more commonly associated with mineral occurrences as gangue or alteration products. Some silicate minerals, such as zircon and spodumene, are ore minerals and represent important sources of metals such as zirconium and lithium, respectively. Others, such as kaolinite, are mined for their intrinsic properties (i.e. as a clay for the ceramics industry).
anionic group. Silicate minerals are generally hard, refractory, and translucent. Most of them cannot be regarded as ore minerals in that they do not represent the extractable part of an ore body, and the list provided below shows only some of the silicates more commonly associated with mineral occurrences as gangue or alteration products. Some silicate minerals, such as zircon and spodumene, are ore minerals and represent important sources of metals such as zirconium and lithium, respectively. Others, such as kaolinite, are mined for their intrinsic properties (i.e. as a clay for the ceramics industry).










