Inevitably, however, new discoveries lead to new questions that are not entirely answerable by the theory, and those questions beget more questions. Soon the model enters crisis mode and begins to drift as scientists seek to adjust it, as little as possible, to account for that which it cannot explain.
Crisis mode is always a fascinating time in science but one that is not for the faint of heart, as doubts about the views of previous generations continue to grow against the old guard’s protestations. But the chaos is ultimately replaced by a paradigm shift, one in which a new consensus model emerges that can explain more than the previous model.
That’s what happened about a decade ago, as the ideas of leading scientists in the aging field began to coalesce around a new model—one that suggested that the reason so many brilliant people had struggled to identify a single cause of aging was that there wasn’t one.
In this more nuanced view, aging and the diseases that come with it are the result of multiple “hallmarks” of aging:
Genomic instability caused by DNA damage
Attrition of the protective chromosomal endcaps, the telomeres
Alterations to the epigenome that controls which genes are turned on and off
Loss of healthy protein maintenance, known as proteostasis
Deregulated nutrient sensing caused by metabolic changes
Mitochondrial dysfunction
Accumulation of senescent zombielike cells that inflame healthy cells
Exhaustion of stem cells
Altered intercellular communication and the production of inflammatory molecules
Researchers began to cautiously agree: address these hallmarks, and you can slow down aging. Slow down aging, and you can forestall disease. Forestall disease, and you can push back death.
Take stem cells, which have the potential to develop into many other kinds of cells: if we can keep these undifferentiated cells from tiring out, they can continue to generate all the differentiated cells necessary to heal damaged tissues and battle all kinds of diseases.
Meanwhile, we’re improving the rates of acceptance of bone marrow transplants, which are the most common form of stem cell therapy, and using stem cells for the treatment of arthritic joints, type 1 diabetes, loss of vision, and neurodegenerative diseases such as Alzheimer’s and Parkinson’s. These stem cell–based interventions are adding years to people’s lives.
Or take senescent cells, which have reached the end of their ability to divide but refuse to die, continuing to spit out panic signals that inflame surrounding cells: if we can kill off senescent cells or keep them from accumulating in the first place, we can keep our tissues much healthier for longer.
The same can be said for combating telomere loss, the decline in proteostasis, and all of the other hallmarks. Each can be addressed one by one, a little at a time, in ways that can help us extend human healthspans.
Over the past quarter century, researchers have increasingly honed their efforts in on addressing each of these hallmarks. A broad consensus formed that this would be the best way to alleviate the pain and suffering of those who are aging.
There is little doubt that the list of hallmarks, though incomplete, comprises the beginnings of a rather strong tactical manual for living longer and healthier lives. Interventions aimed at slowing any one of these hallmarks may add a few years of wellness to our lives. If we can address all of them, the reward could be vastly increased average lifespans.
As for pushing way past the maximum limit? Addressing these hallmarks might not be enough.
But the science is moving fast, faster now than ever before, thanks to the accumulation of many centuries of knowledge, robots that analyze tens of thousands of potential drugs each day, sequencing machines that read millions of genes a day, and computing power that processes trillions of bytes of data at speeds that were unimaginable just a decade ago. Theories on aging, which were slowly chipped away for decades, are now more easily testable and refutable.
Although it is in its early days, a new shift in thinking is again under way. Once again we find ourselves in a period of chaos—still quite confident that the hallmarks are accurate indicators of aging and its myriad symptoms but unable to explain why the hallmarks occur in the first place.
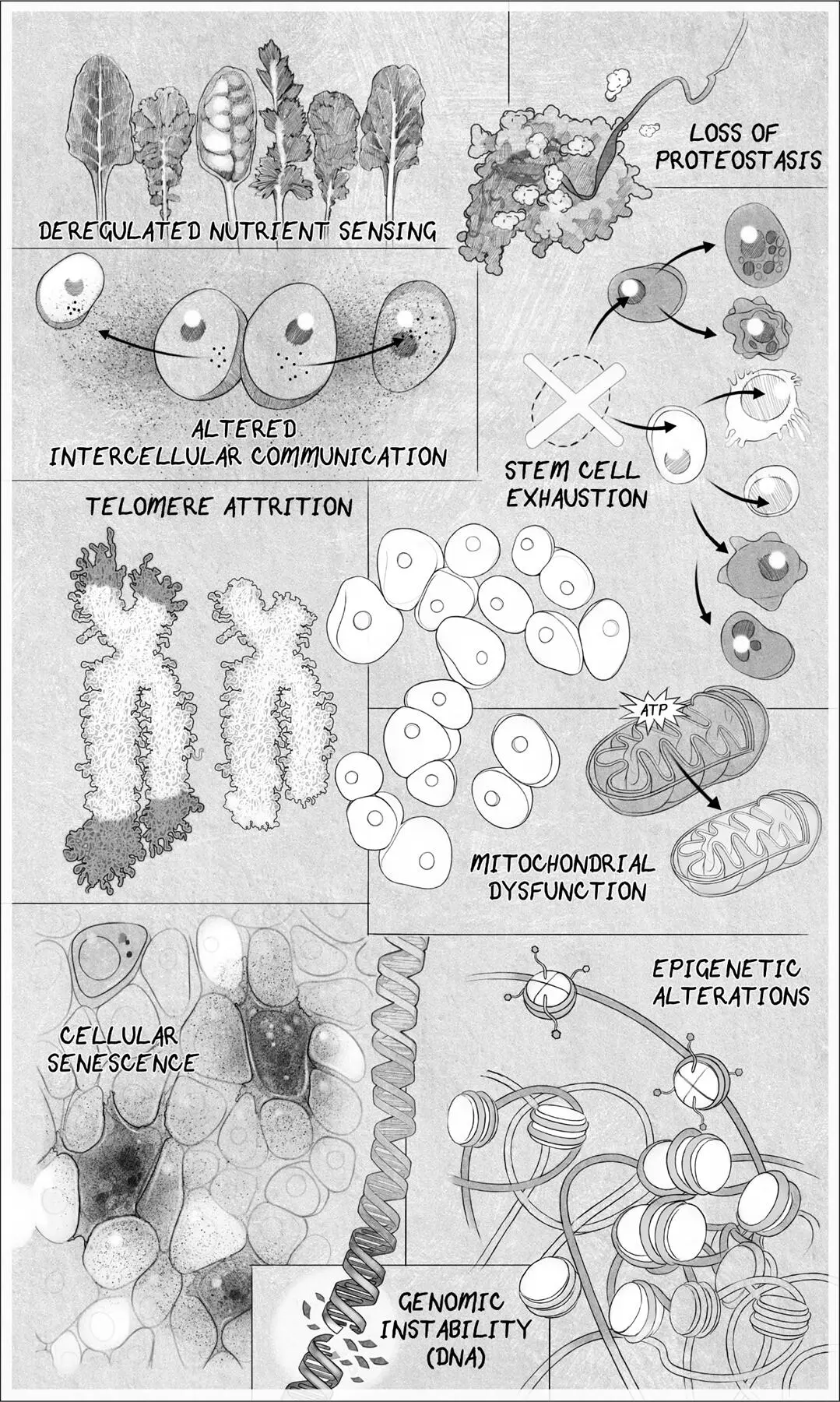
THE HALLMARKS OF AGING.Scientists have settled on eight or nine hallmarks of aging. Address one of these, and you can slow down aging. Address all of them, and you might not age.
It is time for an answer to this very old question.
Now, finding a universal explanation for anything—let alone something as complicated as aging—doesn’t happen overnight. Any theory that seeks to explain aging must not just stand up to scientific scrutiny but provide a rational explanation for every one of the pillars of aging. A universal hypothesis that seems to provide a reason for cellular senescence but not stem cell exhaustion would, for example, explain neither.
Yet I believe that such an answer exists—a cause of aging that exists upstream of all the hallmarks. Yes, a singular reason why we age.
Aging, quite simply, is a loss of information.
You might recognize that loss of information was a big part of the ideas that Szilard and Medawar independently espoused, but it was wrong because it focused on a loss of genetic information.
But there are two types of information in biology, and they are encoded entirely differently. The first type of information—the type my esteemed predecessors understood—is digital . Digital information, as you likely know, is based on a finite set of possible values—in this instance, not in base 2 or binary, coded as 0s and 1s, but the sort that is quaternary or base 4, coded as adenine, thymine, cytosine, and guanine, the nucleotides A, T, C, G of DNA.
Because DNA is digital, it is a reliable way to store and copy information. Indeed, it can be copied again and again with tremendous accuracy, no different in principle from digital information stored in computer memory or on a DVD.
DNA is also robust. When I first worked in a lab, I was shocked by how this “molecule of life” could survive for hours in boiling water and thrilled that it was recoverable from Neanderthal remains at least 40,000 years old. 23The advantages of digital storage explain why chains of nucleic acids have remained the go-to biological storage molecule for the past 4 billion years.
The other type of information in the body is analog .
We don’t hear as much about analog information in the body. That’s in part because it’s newer to science, and in part because it’s rarely described in terms of information, even though that’s how it was first described when geneticists noticed strange nongenetic effects in plants they were breeding.
Today, analog information is more commonly referred to as the epigenome , meaning traits that are heritable that aren’t transmitted by genetic means.
The term epigenetics was first coined in 1942 by Conrad H. Waddington, a British developmental biologist, while working at Cambridge University. In the past decade, the meaning of the word epigenetics has expanded into other areas of biology that have less to do with heredity—including embryonic development, gene switch networks, and chemical modifications of DNA-packaging proteins—much to the chagrin of orthodox geneticists in my department at Harvard Medical School.
In the same way that genetic information is stored as DNA, epigenetic information is stored in a structure called chromatin. DNA in the cell isn’t flailing around disorganized, it is wrapped around tiny balls of protein called histones. These beads on a string self-assemble to form loops, as when you tidy your garden hose on your driveway by looping it into a pile. If you were to play tug-of-war using both ends of a chromosome, you’d end up with a six foot-long string of DNA punctuated by thousands of histone proteins. If you could somehow plug one end of the DNA into a power socket and make the histones flash on and off, a few cells could do you for holiday lights.
Читать дальше













