In 2011, cell‐free DNA screening (also known as noninvasive prenatal testing or noninvasive prenatal screening) became clinically available as a screening test for aneuploidy. This screening test relies on the analysis of cell‐free DNA (cfDNA) fragments in the maternal circulation. After 10 weeks of gestation, approximately 10–15% of the cfDNA in the maternal serum is of placental origin and therefore reflects the fetal DNA. Clinical testing measures the chromosomal contribution of the cfDNA in the maternal circulation to determine whether there is over‐ or underrepresentation of targeted chromosomes. Different laboratories use different approaches, including massively parallel shotgun sequencing (MPSS), a targeted microarray approach, or targeted sequencing using single nucleotide polymorphisms (SNPs); performance for aneuploidy screening is generally comparable between platforms. Standard cfDNA screening tests for trisomies 13, 18, and 21, and can also assess the sex chromosomes to determine fetal sex and, in some cases, screen for sex chromosomal aneuploidy.
The accurate performance of cfDNA screening depends on the presence of adequate fetal (placental) cfDNA, referred to as the “fetal fraction.” In some laboratories, a result is not provided when the fetal fraction falls below a prespecified level; this cut‐off is typically about 4%. Early gestational age, increasing maternal body mass index, and fetal aneuploidy are associated with a lower fetal fraction and increase the chances of a failed test.
Studies of test performance for cfDNA screening report a >99% detection rate for fetal trisomy 21 and 98% detection for trisomy 18 with a combined false‐positive rate (FPR) of 0.13–0.25%. Because trisomy 13 is a rare disorder, data are far more limited but reported detection rates vary from 40% to 100% in individual studies. The detection rate of sex chromosome aneuploidy is also difficult to determine due to limited data.
Importantly, these data were calculated for patients with a reported result, and as many as 3–4% of samples result in test failure. Test failure, particularly in the setting of low fetal fraction, is associated with an increased risk of aneuploidy and patients should be counseled accordingly and offered follow‐up testing.
While cfDNA screening has excellent performance in detection of trisomy 21, both false‐positive and false‐negative results can occur, particularly with low fetal fraction. The presence of mosaicism or a vanishing twin may result in false‐positive cfDNA results. Standard cfDNA screening tests do not provide risk assessment for other chromosomal, genetic, or structural disorders. Some laboratories offer expanded cfDNA panels to test for chromosomal microdeletions, rare autosomal trisomies, or genome‐wide copy number variants. Such tests have not been clinically validated, performance characteristics are unknown, and these are generally not recommended at the present time.
First‐trimester combined screening
The ability to provide an accurate, patient‐specific, risk assessment for fetal trisomy 21 during the first trimester is an established part of routine clinical practice. This allows patients the option of CVS to confirm or exclude fetal aneuploidy, and the possibility of pregnancy termination earlier in gestation. Such patient‐specific risk estimation is currently most commonly performed using a combination of maternal age, sonographic measurement of nuchal translucency (NT), and assay of two maternal serum markers – pregnancy‐associated plasma protein A (PAPP‐A) and either the free beta‐subunit (fβ) or the intact molecule of human chorionic gonadotrophin (hCG).
Nuchal translucency sonography
Nuchal translucency refers to the normal space that is visible between the spine and overlying skin at the back of the fetal neck during first‐trimester sonography ( Figures 5.1and 5.2). The larger this space, the higher the risk for trisomy 21, while the smaller the space, the lower the risk for trisomy 21. Measurement of the NT between 11 weeks and 3 days and 14 weeks and 2 days of gestation (45–84 mm) has been shown to be a useful sonographic marker for trisomy 21. Table 5.1describes the components of a standardized NT sonographic protocol.
Nuchal translucency sonography can be technically challenging to master and it requires considerable effort to maintain quality over time. Given the importance of maintaining such accuracy, sonographers and physicians who provide this form of screening should be credentialed and enrolled in an ongoing quality assurance program. Examples of such QA programs include the Nuchal Translucency Quality Review (NTQR) managed by the Perinatal Quality Foundation in the US ( www.ntqr.org) and the Fetal Medicine Foundation in Europe ( www.fetalmedicine.org).
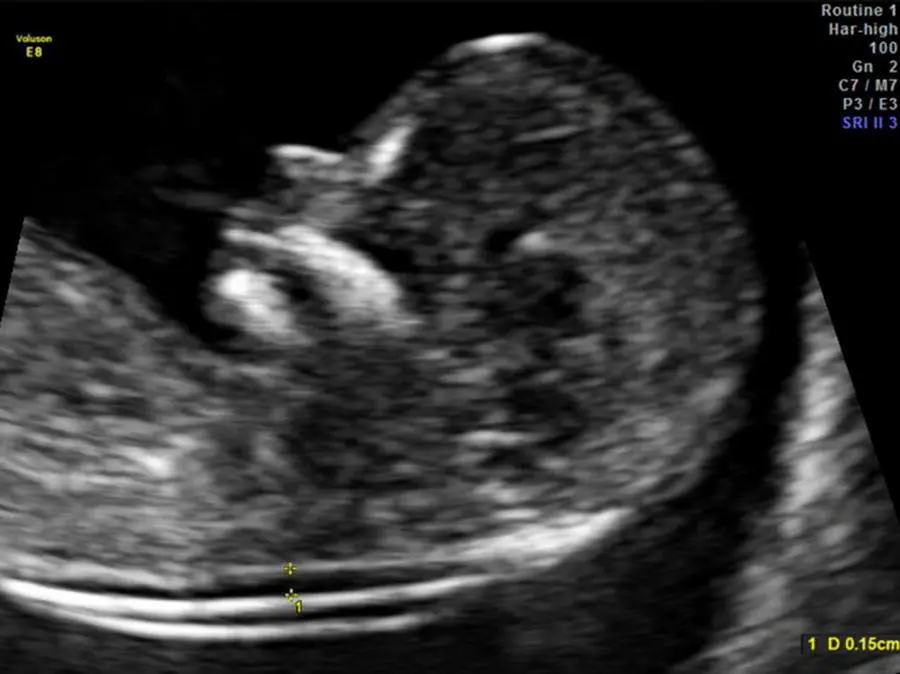
Figure 5.1 Nuchal translucency (NT) ultrasound measurement at 13 weeks’ gestation in a chromosomally normal fetus, measuring 1.5 mm. Various features of good NT ultrasound technique are evident in this image: adequate image magnification, midsagittal plane, neutral neck position, inner to inner caliper placement perpendicular to the fetal body axis, and separate visualization of the overlying fetal skin and amnion.
Source: Mary E. Norton, MD.
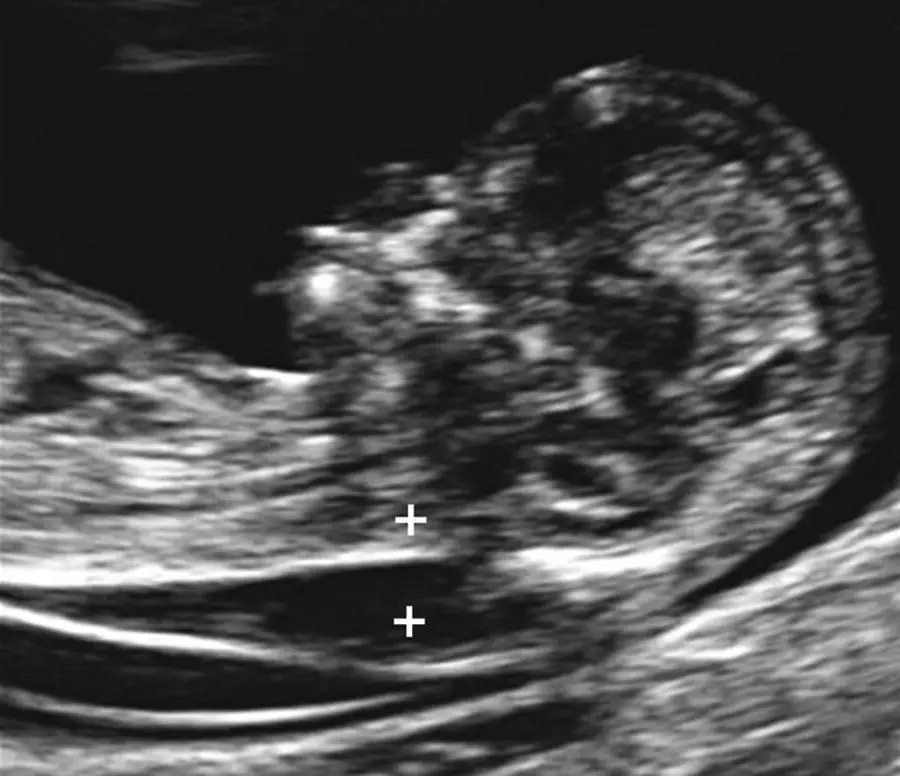
Figure 5.2 Increased nuchal translucency measurement at 13 weeks’ gestation in a fetus with Down syndrome.
Source: Mary E. Norton, MD.
Table 5.1 Nuchal translucency (NT) measurement criteria of the Nuchal Translucency Quality Review (NTQR) Program
| 1 |
Fetal head, neck, and upper thorax should fill the majority of the image (>50%) |
| 2 |
Image should be optimized so the NT lines are thin and clear |
| 3 |
Fetus should be examined in a midsagittal plane |
| 4 |
Fetal neck should be in a neutral position |
| 5 |
Fetus should be observed away from the amnion |
| 6 |
The “+” calipers should be used |
| 7 |
Calipers should be placed on the echogenic inner borders of the nuchal membranes with none of the horizontal crossbars protruding into the translucent NT space |
| 8 |
Calipers should be placed perpendicular to the long axis of the fetal body |
| 9 |
At least three nuchal translucency measurements should be obtained and the maximum acceptable measurement should be used |
| 10 |
The ALARA (as low as reasonably achievable) criteria should be followed and the thermal index for bone (TIB) set with an output standardized display of ≤0.7 |
First‐trimester PAPP‐A and hCG
[fo]Maternal serum levels of PAPP‐A are approximately 50% lower, and hCG levels (either total hCG or fβhCG) approximately twice as high, in trisomy 21 pregnancies compared with euploid pregnancies at 10–14 weeks of gestation, and these analytes can be used for assessment of trisomy 21 risk in the first trimester. The combination of maternal age, NT sonography, PAPP‐A, and hCG is referred to as first‐trimester combined screening and will detect about 85% of cases of trisomy 21, at a 5% false‐positive rate, between 10 and 14 weeks of gestation.
Читать дальше














