Using renewable packaging materials is not a novel strategy, since some ancient human practices of food preservation were based on renewable raw materials. For instance, fruit waxing has been used since the twelfth century in China, meat larding has been employed in England since the sixteenth century, as well as wrapping ground meats or vegetables with soy films in the Orient [11]. Currently, considerable progress has been made in developing packaging based on renewable materials, among them, films and coatings based on biopolymers derived from marine sources [12]. Thereby, by‐products from seafood industry can be used in the development of environmentally friendly packaging solutions to prevent food spoilage and extend shelf life [13].
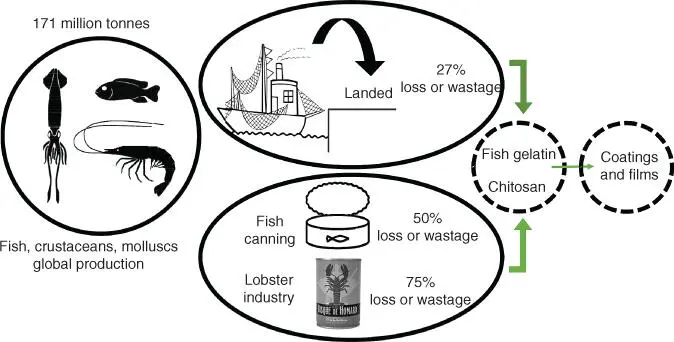
Figure 2.1 Fishery production, wastage, and possible solutions.
The global fish production (fish, crustaceans, mollusks, and other aquatic animals, including fisheries and aquaculture) peaked at about 171 million tons in 2016 [14], with an estimated loss or wastage between landing and consumption of 27% of landed fish, crustaceans, and mollusks ( Figure 2.1). Additionally, high amounts of solid waste generated in fishery industries must be considered; for instance, in fish canning operations, the amount of waste can be as high as 50% by weight of raw products [15]. In all these fish by‐products (skin, bones, scales, tendons), there is a considerable amount of collagen, a fibrous protein with a structural function [16, 17]. Carrying out thermal denaturation or partial hydrolysis of collagen, gelatin is obtained [18], whose properties differ between fish species. In general, cold‐water fish gelatins have relatively lower gel strength than warm‐water fish gelatins due the lower content of imino acid residues [19]. In this regard, gelatin from salmon skin (cold‐water fish) has 166 residues per 1000 total amino acid, whereas gelatin from sea bass skin (warm‐water fish) contains around 198–202 residues per 1000 total amino acid [20].
In the case of crustacean and mollusks by‐products, shrimp and crab shells can be used to obtain chitin [21]. As an example, lobster processing industry by‐products account for around 75% by weight of the starting material ( Figure 2.1). Chitin has a similar structural function to that of collagen. Chitin is the second most abundant natural polymer after cellulose, and it is usually transformed into chitosan, a water‐soluble polymer, to broaden its application. This transformation process is based on chitin deacetylation under alkaline conditions [22–24]. It must be considered that the functional properties of chitosan depend on structural features, such as average molecular weight and degree of deacetylation [25].
This chapter provides an overview of packaging films and coatings based on biopolymers derived from marine sources, with emphasis on fish gelatin and chitosan materials. The techniques used for extraction of biopolymers, preparation methods of films and coatings, and their characterization, together with diverse novel techniques employed for extending food products shelf life, will be described in this chapter.
2.2 Fish Gelatin Films and Coating
2.2.1 Collagen and Gelatin Extraction
Collagen can be obtained from fish skin, scales, and bones, which are cleaned and reduced in size to facilitate the extraction [26]. The extraction process from fishery wastes consists of two main steps: pretreatment of raw materials and collagen extraction. In general, alkaline pretreatment is carried out to remove impurities such as non‐collagenous proteins and lipids, as well as to increase the quality of the final extracted collagen [27]; and it can be carried out using a strong alkali, such as sodium hydroxide [20, 28] or calcium hydroxide [29]. Sometimes, to remove the lipids, alcohols such as ethanol [30, 31], isopropanol [32], or butyl alcohol [33, 34] are also used. Additionally, the demineralization of the raw materials can be carried out using ethylenediaminetetraacetic acid (EDTA); thus, calcium or other inorganic materials are removed [35]. After that, the extraction of collagen is usually carried out.
There are numerous methods reported for collagen extraction, based on three main extraction processes: extraction of salt‐soluble collagen (SSC), acid‐soluble collagen (ASC), and enzyme‐soluble collagen (ESC). These extraction methods directly affect collagen properties and yield [36], and depend on factors such as fish species and age [37]. It is worth noting that all procedures within collagen extraction are performed at low temperature (∼4 °C) for 24–48 hours. Although an increasing extraction temperature and time can offer a higher collagen yield, it may not be desirable due to collagen degradation [38].
Regarding extraction in NaCl solution (SSC extraction), lower yields are obtained compared with ASC and ESC extractions [39]; hence, this method is not widely utilized and acid and/or enzymatic extractions are preferred. In terms of acidic extractions, the use of acetic acid medium for extraction and isolation of collagen from by‐products such as sabalo skin [26] and sea bass scales [40] has been reported. In addition to acetic acid, collagen can also be extracted using other organic acids, such as citric acid [32], or inorganic acids such as hydrochloric acid [41] and phosphoric acid [42]. However, it must be considered that the solubility of collagen in acidic media is limited owing to many interchain cross‐links, covalent bonds formed via the condensation reaction of aldehydes with lysine and hydroxylysine at the telopeptide region [43]. Moreover, habitat and age of animals or collagen by‐product source affect directly the content of keto–imine bonds and, hence, the solubility in acidic media [44]. In order to improve solubility and achieve higher yield, extraction parameters can be changed, including acid concentration, ratio of acid solution/raw materials, extraction temperature and time; however, these parameters must be controlled in order to avoid collagen degradation [45]. Depending on the degree of collagen aggregation, the extraction process could also include the use of proteolytic enzymes [46–48], which together with an acidic solution might lead to an increase in the yield, by combining biological and chemical pretreatments. Diverse enzymes have been employed, among them, trypsin and pepsin digestive proteases [49] and bacterial collagenases [27]. Indeed, the most commonly employed enzyme is pepsin and, in this sense, the resultant pepsin‐extracted collagen is called pepsin‐soluble collagen (PSC) [50]. This digestive protease can remove non‐collagenous proteins and increase the purity of collagen. Thus, non‐collagenous proteins can be hydrolyzed by pepsin and removed using salt precipitation and dialysis. Additionally, pepsin can also hydrolyze collagen telopeptides, contributing to the treated collagen solubilization in acid media and, thereby increasing the yield of acid‐soluble extraction [36]. Several studies have shown an increase in the acidic extraction yield with the aid of pepsin [40, 51, 52]. It is worth noting that commercial pepsin is commonly obtained from porcine gastric mucosa; however, in order to avoid some religious restrictions a huge range of proteolytic enzymes, including pepsin, can be extracted from fish viscera [34]. Furthermore, the choice of suitable enzymes and physiochemical conditions for the enzymatic reaction, such as solution pH, temperature, hydrolysis time, and enzyme concentration, must be optimized for the maximum activity. In recent years, other techniques have been used to improve SSC, ASC, and ESC extraction methods, among them, ultrasonic treatments and homogenization‐aided methods. Zou et al. [53] worked with ultrasonic power of 200 W having a single frequency of 24 kHz to obtain collagen extracted from calipash of soft‐shelled turtle. On the other hand, Tan and Chang [41] successfully extracted collagen from catfish skin by mixing catfish skins, hydrochloric acid, and pepsins at 7000 rpm for five minutes until homogenization and then the mixture was stirred for one hour at 4 °C. After extraction processes, some final steps must be carried out. Thus, collagen is usually recovered using salt precipitation, centrifugation, dialysis, and freeze‐drying. Generally, collagen solution is precipitated using NaCl. The salt concentrations employed for collagen precipitation can be adjusted to maximize the collagen recovery and removal of impurities. Then, centrifugation (around 10 000–20 000 rpm) is used to collect the precipitated collagen. The resultant precipitate is dissolved in acetic acid prior to dialysis against distilled water. The dialysate is finally freeze‐dried and the obtained collagen powder is stored [43].
Читать дальше












