Sarah Walter and Marcelo E. Bigal (Antiva Biosciences and Ventus Therapeutics) describe CGRP (calcitonin gene‐related peptide) inhibitors for the treatment of migraine, which represent a new class of drugs consisting of both small‐molecule drugs and biologics.
Takehisa Kitazawa, Koichiro Yoneyama , and Tomoyuki Igawa (Chugai Pharmaceuticals) provide a case study of emicizumab, a humanized bispecific antibody to coagulation factors IXa and X that also possesses factor VIII cofactor activity. Emicizumab (HEMLIBRA™) was approved by US FDA in 2017 for treatment of hemophilia A.
Zenon D. Konteatis and Zhihua Sui (Agios Pharmaceuticals) describe the discovery and development of ivosidenib (Tibsovo™), which was approved by US FDA in 2019 for newly diagnosed acute myeloid leukemia with a susceptible IDH1 mutation.
Christopher T. Brain, Rajiv Chopra, Sunkyu Kim, Steven Howard , and Moo Je Sung (Novartis) recount the discovery of ribociclib (Kisqali™), a CDK4/6 inhibitor for the treatment of HR positive/HER2 negative advanced brain cancer. Ribociclib was approved by the US FDA in 2017 for use in combination with an aromatase inhibitor.
The editors and authors thank Wiley‐VCH and personally Dr. Frank Weinreich and Katherine Wong for the excellent collaboration.
János Fischer
Budapest
Wayne E. Childers
Philadelphia
Christian Klein
Zürich
June 2020
Part I General Aspects
1 Drug Discovery in Academia
Oliver Plettenburg1
1Helmholtz Zentrum München (GmbH), German Research Center for Environmental Health, Institute of Medicinal Chemistry, Ingolstädter Landstr. 1, D‐85764, Neuherberg, Germany
2Leibniz Universität Hannover, Center for Biomolecular Research, Institute of Organic Chemistry, Schneiderberg 1b, D‐30167, Hannover, Germany
It is estimated that the global pharmaceutical industry invested more than US$ 1.36 trillion in the decade from 2007 to 2017, and predicted annual spending is assumed to totally sum up to 181 billion for the period to 2020 [1]. At the end of 2019, the 10 largest pharmaceutical companies represented a market capitalization of approximately US$ 1.68 trillion [2].
The tremendous advances in science starting in the 1990s stipulated hopes that the discovery of new medicines would soon turn into an engineerable process. The decryption of the human genome provided a plethora of new target opportunities for exploitation, and the availability of large screening collections, efficient miniaturized high‐throughput screening technologies, and computer‐assisted methods for hit generation suggested that generation of reasonable lead structures should be feasible for many of these targets. Furthermore, cellular models for early prediction of metabolic liabilities and toxicological risks enhanced the optimization of drug‐like properties. However, after 30 years, these hopes did not turn into reality; the number of approved drugs remained approximately constant, at least for the period from 1989 to 2013. In 2019, the Food and Drug Administration (FDA) approved 47 new drugs, 9 of which are biologics ( Figure 1.1) [3]. It is an interesting observation that despite the trend to focus research on biologics and small‐molecule drug business was said to be dead for several years, the fraction of annual new biological drug approvals is still stagnating at about 25 %.
In an article published in 2011, Stevens [4] analyzed the contributions of publicly funded organizations to current approval rates over a period of 40 years. It is remarkable to note that about 9 % of all approvals (143/1541) were enabled or at least facilitated by public funding. If one compares the contributions for new molecular entities, the rate rises to 13.3 % (64 out of 483). For new molecular entities that have been granted priority review, the report cites an impressive 21.1 % (44 out of 209). In a recent study, Nayak et al. [5] confirmed the significance of pharmaceutical research driven by universities and clinical centers. They thoroughly analyzed FDA drug approvals between 2008 and 2019, considering also patent information. Among the 248 approvals of new molecular entities, they identified significant contributions by publicly funded organizations for 62 (25 %) of them. It is puzzling that pharmaceutical ventures with their highly skilled scientists and an infrastructure that is capable of accessing virtually unlimited funds dedicated solely to the purpose of drug discovery did not perform better than these figures tell us. This is even more surprising in light of the fact that provision of new drugs to the pipeline is obviously a vital task in order to maintain the company going in the future and patent lifetime of approved therapies is clearly very limited.
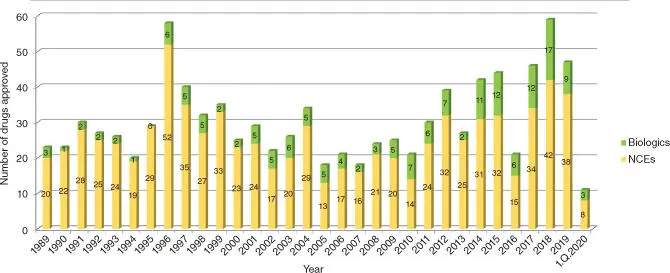
Figure 1.1 FDA drug approvals from 1990 to 2019.
Source: Data from Mullard [3].
Independent development of a drug to a marketed product is clearly out of scope for any academic. It is estimated that out of pocket costs for approval of a single drug can amount to US$ 1.3 billion, with the majority of this budget being consumed by clinical trials. Also the process needs oversight and management by experienced clinical scientists to optimally set up the studies in order to ensure that a potential beneficial outcome will not be a victim of an underpowered study group or that the selection of the patient population was not optimal.
However, when the clinical trial starts, the selection process of the therapeutic moiety is already completed and the decision on target and approach is taken, from that point on it is the task of the clinicians to see if the generated hypothesis will hold true.
However, academics provide important contributions to drug discovery, using their specific strengths. These can be based on curiosity, expert knowledge in specific areas, exploitation of surprising findings, stimulating follow‐up research, and interdisciplinary research resulting from different academic laboratories teaming up, for instance. Different examples of how these specific strengths can lead to successful drug discovery will be discussed throughout this chapter.
One contribution ideally suited for academic research is the quest for new indications.
As approved drugs are openly commercially available, researchers, particularly scientists in clinical centers, can – based on patient derived data – generate hypotheses and probe them in a straightforward manner. In this context drug repurposing has attracted a lot of attention as the approach is very straightforward, and the resulting drug has already been demonstrated to be safe, bioavailable, and well tolerated in humans.
Often, this approach is guided by careful observation of disease‐accompanying factors and interpretation of the underlying pathology. In particular, changes of symptoms in patients suffering from more than one disease may provide interesting starting points for developing new hypotheses. An example is rituximab, which first was developed for the treatment of cancer. Its discovery will be discussed in more detail during the course of the chapter. Edwards et al. proposed that self‐perpetuating B‐lymphocytes may play a key role in driving progression of rheumatoid arthritis (RA) and autoimmune diseases [6]. They hypothesized that a CD20 (cluster of differentiation 20) targeted therapeutic, capable of specifically depleting this population of B‐cells, may represent an interesting therapeutic option. In 1999, a first case report of a patient suffering from non‐Hodgkin's lymphoma in association with inflammatory arthropathy appeared [7]. Within weeks of treatment with a monoclonal anti‐CD20 antibody, significant improvement of joint pain was observed, and three months later, the patient was virtually symptom‐free and capable of walking distances of 5 miles per day. In a following phase 2 study, positive results of rituximab in patients with RA were demonstrated, [8] followed by further trials. After being able to demonstrate convincing beneficial effects, rituximab was approved for treatment of RA in combination with methotrexate in 2006.
Читать дальше













