In addition to the importance of epigenetic mutations in PTCL lymphomagenesis, and the therapeutic rationale, recent evidence suggests that surveillance of these mutations may be useful in the monitoring of TCL response to therapy. Cell‐free DNA (cfDNA) is increasingly being validated for the purpose of detection of minimal residual disease, as well as for the genetic profiling of a known malignancy. Mutations in genes with established roles in epigenetic regulation such as TET2, DNMT3A, and IDH2 have been found to be 83% concordant between cfDNA and tissue biopsy samples in patients with AITL, and may emerge as a potential marker for detecting minimal residual disease [58].
Epigenetic Changes Within Specific T‐cell Lymphoma Subtypes
PTCL oncogenesis is a complex process thought to comprise two distinct components: one involving the dysregulation of T‐cell receptor signaling pathways intrinsic to malignant T cells, the other involving the interplay between the malignant cell and the non‐neoplastic tumor microenvironment. In addition, in select PTCL subtypes, the neoplastic transformation can be driven by viruses and chronic inflammation. In addition to the extensive epigenetic dysregulation discussed above, it is now becoming clear that a host of other molecular derangements can contribute to dysregulation of the PTCL epigenome, including but not necessarily limited to chromosomal translocations, insertions, deletions, point mutations, can result in unique fusion proteins, constitutive activation of driver pathways and gene loss [59, 60].
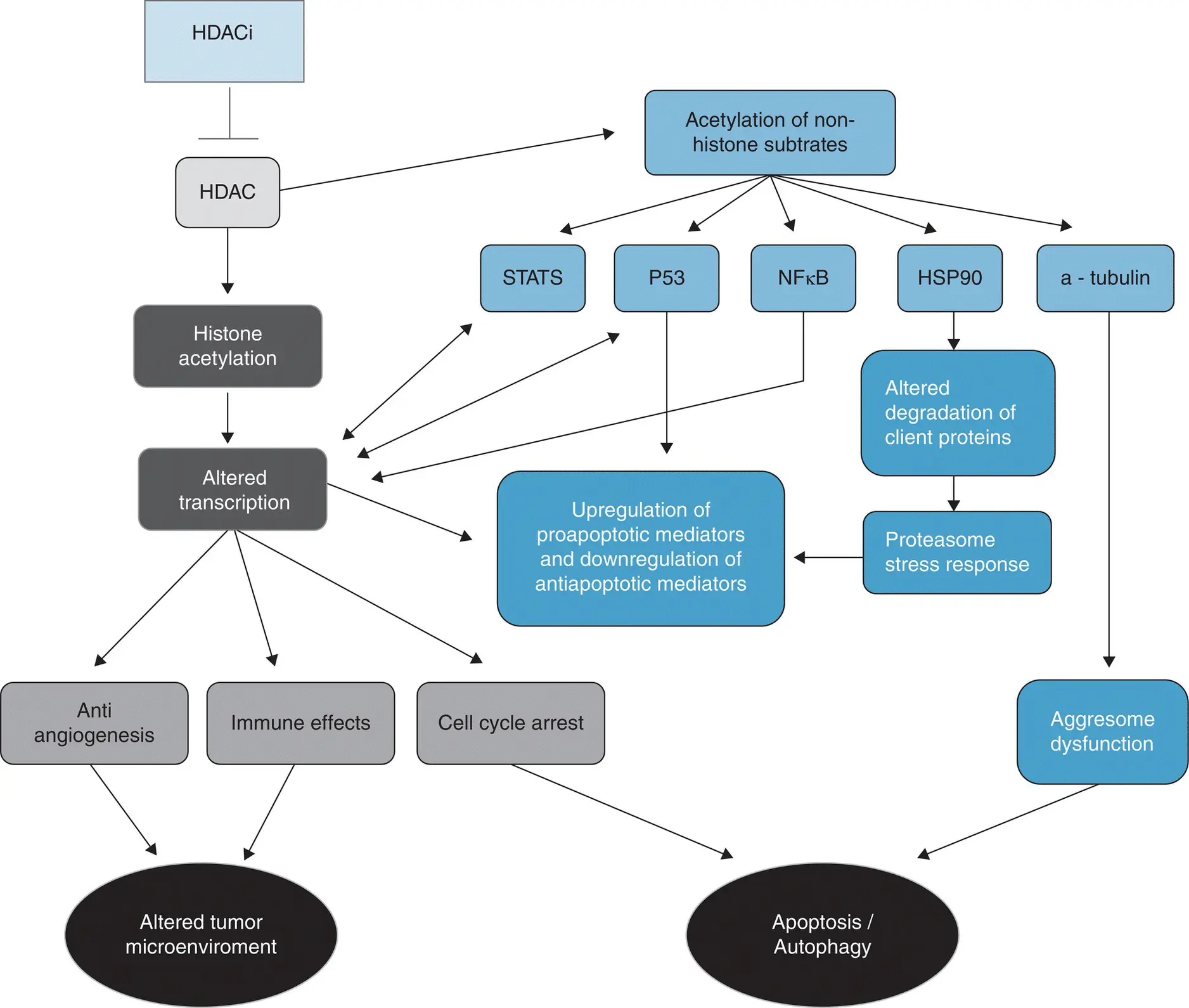
Figure 3.2 A simplified schematic of the action of histone deacetylase inhibitor.
Peripheral T‐cell Lymphoma Not Otherwise Specified
Epigenetic mutations are less frequent in PTCL‐NOS than in AITL (see section 3.3.2). There are reports of mutations in epigenetic regulators including KDM6A, MLL2, TET2 , and DNMT3 which control genes involved in various signaling pathways including ZAP70, CHD8, APC , and TRAF3 . Most importantly, a subgroup of PTCL is now defined as PTCL with T follicular helper (Tfh) cell phenotype (see below) and is now a separate category to PTCL‐NOS as defined by the World Health Organization classification.
Angioimmunoblastic T‐cell Lymphoma and Peripheral T‐cell Lymphoma with T Follicular Helper Phenotype
The clinical presentation of these two subtypes is nodal with a plethora of associated symptoms including arthralgias, skin rashes and autoimmune phenomena that can be explained by the role of Tfh cells in the regulation of B cells. The role of the Epstein–Barr virus (EBV) in the pathogenesis of AITL is still not defined but progression to EBV positive DLBCL is a well‐recognized occurrence in AITL [61]. The Tfh cell is the putative cell of origin for these two subtypes of PTCL. Normal Tfh cells are located in the germinal center of the lymph nodes, driven there by CXCR5 expression where they push germinal center B cells toward differentiation into plasma cells and memory cells. The two histological subtypes are distinguished by the presence (AITL) or absence (PTCL with Tfh phenotype) of abundant endothelial venules in the tumor sample. Both subtypes require that the neoplastic cells express at least two or more markers of Tfh cells such as PD‐1, BCL‐6, CXCL13, CD10, ICOS, and CXCR51 [62]. EBV‐infected cells are present in many cases of AITL. Higher EBV viral loads in tissues have been found to correlate with progression of disease and B‐cell clonality. Human herpesvirus 6B is also detected by PCR in almost half the cases of AITL. These viral infections reflect the immune deregulation seen in patients with AITL [63]. The most common cytogenetic abnormalities are trisomy 3, trisomy 7 and an additional X chromosome. Other genetic abnormalities include recurrent trisomy 5 often concurring with trisomy 21 [64]. Gene expression profiling of AITL identifies a pathogenic pathway that includes nuclear factor kappa B (NF‐κB signaling, interleukin 6 signaling, and transforming growth factor beta pathways and can be differentiated from PTCL‐NOS [60]. Small focal deletions show enrichment in genes resulting in dysregulation of the PI3K‐AKT‐mTOR pathway, similar to that demonstrated in the murine models discussed above. Half of AITL‐ and TFH‐derived PTCLs have mutations in the T‐cell receptor signaling pathways, including of CD28, FYN, PLCG1, CARD11, PI3K, CTNNB1 , and GTF21 [8, 60, 65–68].
These two subtypes exhibit the highest degree of epigenetic dysregulation within the PTCL subgroup and may be the most susceptible to epigenetic therapies [34, 42]. Mutations in IDH1 and IDH2 , which encode cytosolic and mitochondrial forms of IDH, lead to loss of normal catalytic activity as well as production of 2‐hydroxyglutarate. Downstream effects include inhibition of the TET family of DNA hydrolases resulting in abnormal histone and DNA methylation that leads to T‐cell transformation. IDH2 mutations are identified in about a third of AITL cases and some cases of PTCL. TET2 inactivating missense or nonsense mutations or insertions/deletions have been reported in up to 85% of AITL resulting in DNA hypermethylation, which also appears to have effects on other proteins including HDAC1/2. Only IDH2 codon 172 mutations have been observed in AITL and occur with TET2 mutations.
DNMT 3 loss of function is reported in 10–25% cases of AITL, 80% of which also have TET2 mutations confirming a strongly dysregulated epigenome in AITL. Epigenetic mutations in TET2 , DNMT3 , and IDH2 are strongly associated with the RHOA G17V mutation. The RHOA G17V mutation is seen exclusively in the background of TET2 mutations with or without IDH2 mutations in 70% of AITL patients. These mutants do not bind GTP and disrupt the important RhoA signaling. The RHOA G17V mutation results in increased AKT activity through several interactions that include VAV1, ROCK1 and 2 and PTEN. A subset of IDH2 mutated cases harbor both TET2 and RhoA [69].
Epigenetic therapies deserve a special mention for this subtype. Single‐agent use of the HDACi romidepsin and belinostat is approved for relapsed PTCL. As discussed above, epigenomic dysfunction in PTCL is quite extensive and seems to have the highest impact in AITL. Interestingly, romidepsin and belinostat have been reported to have an overall response rate of 30% [23] 45% in AITL in the pivotal trials, which is higher than reported for other TCLs [70, 71]. While some have interpreted these data to imply a greater level of vulnerability of AITL to HDAC inhibitor, the data should be viewed with caution given the very small numbers of this subset in those studies, and the absence of any randomized data for these drugs in this setting.
Similarly, HMA are only recently being evaluated in the treatment of AITL, given the preponderance of mutations affecting genome methylation in this particular subtype. Delarue et al. reported on the use of 5‐azacitidine in 19 patients with relapsed/refractory PTCL. Rituximab was added if the patients were positive for EBV. The overall response rate was 75% in the 12 patients with AITL compared with 15% for the other subtypes (limited only to PTCL‐NOS), with a complete response rate 42% among patients with AITL. Mutational analysis showed that all responding patients had a TET2 mutation [72]. Combinations of romidepsin and 5‐azacitadine have reported even higher response rates of 83% with a complete response of 50% among all patients with PTCL, and an even higher overall response rate among patients with AITL [52]. This combination is now being tested in a larger study with correlatives to study possible biomarkers that may predict response in the future. Other strategies being explored include inhibitors of IDH2 specific for the R172 codon and PI3K inhibitors.
Читать дальше













