The question is why do TCLs and other hematologic malignancies show a selection pressure for specific patterns of epigenetic modification? It is well recognized that epigenetic modulation is responsible for sustaining the adaptive transcriptional memory for both central and tissue resident memory T cells [25]. Specifically, this provides the means for different T helper subsets to transcribe inducible genes more rapidly [26].
A key clue that the TCLs represent an “epigenetic disease” came from the empiric finding that HDACi exhibit consistent and reproducible activity (e.g., a response rate of approximately 25%) across all TCL subtypes. While only one in four patients can expect to respond to HDACi, the duration of response seen across these drugs is impressive, typically more than one year. Preclinical studies have confirmed that this vulnerability to HDACi can be exploited in combination. Many studies have now established that the HDACi exhibit potent synergy with a host of other drugs known as “active” in TCL, including HMA, pralatrexate, proteasome inhibitors, and aurora A kinase inhibitors, and could serve as a cornerstone for future platform development [27–33].
Cutaneous TCL (CTCL) was the first malignancy for which HDACi were approved. CTCL is a disease where a panoply of epigenetic abnormalities might explain why HDAC inhibition is associated with clinical benefit in this disease [5]. While mutations in DNMT3A, IDH2, TET2, MLL2, KMT2A, KDM6A, CREBBP , and EP300 genes have been well recognized for years, it remains unclear whether any of these genetic factors portend differential response to HDAC inhibitors [3, 6–10].
DNMT3A functions as a DNA methyltransferase catalyzing cytosine methylation of CpG islands in promoters leading to transcriptional silencing. While mutations in DNMT3A have been identified in about 11–33% of patients with PTCL due to missense or nonsense mutations [34, 35], the mutations frequently coexist with mutated TET 2, which may ultimately lead to transcriptional repression [36].
The TET2 gene encodes an alpha‐ketoglutarate dependent dioxygenase, which converts 5mC to 5‐hydroxymethylcytosine (5hmC), 5‐formylcytosine (5fC), and 5‐carboxylcytosine (5caC) [37–39]. Oxidation of 5mC is part of a demethylation pathway that influences transcriptional activation, where hypermethylation leads to silencing of gene expression, while hypomethylation leads to gene expression. The TET family of proteins are also known to be important in T‐cell differentiation, where loss of function mutations lead to TCL with follicular helper cell‐like features [40–42]. Interestingly, TET2 mutations are seen in up to 70% of PTCL patients. Specifically, it is estimated that TET mutation are found in 42–83% of patients with AITL, 28–48.5% of patients with PTCL not otherwise specified (PTCL‐NOS), and 10% of patients with adult T‐cell leukemia/lymphoma (ATLL) [34–36, 40–43]. Patients with PTCL‐NOS who carry the TET2 mutation often present with a follicular helper type of PTCL, a newly described entity that exhibits many similarities with AITL. Moreover, there can be interactions between the various epigenetic modulators. For example, wild‐type DNMTA3 binding occurs when there is less CpG density compared to TET1 binding, which occurs at a relatively higher CpG density. Both affect the polycomb repressive complex 2 (PCR2)‐mediated methylation of lysine 27 of histone H3 (H3K27), which leads to enrichment of trimethylation at H3K27 and ultimately influences gene expression [44].
Mutations in IDH2 , especially at the R172 residue, have also been identified. Mutations in IDH2 alter the catalytic reactions of the Krebs cycle. Wild‐type IDH converts isocitrate to α‐ketoglutarate, a key co‐factor in the oxidative demethylase reactions which remove methyl groups from DNA. Mutant IDH2 converts isocitrate to 2‐hydroxyglutarate, which is an oncogenic metabolite that cannot function as an obligatory cofactor of TET catalytic functions [34, 45–51]. Mutations in IDH2 and TET2 reduce 5hmC levels due to global hypermethylation of promoters and 5′‐cytosine‐phosphate‐guanine‐3′ (CpG) islands (i.e. leading to transcriptional repression and gene silencing), likely contributing to peripheral T‐cell lymphomagenesis [49, 50]. While there are only modest data for the role of HMA in TCL, emerging data suggest that they do have marked single‐agent activity in AITL and appear to synergize with HDACi in both preclinical and clinical PTCL experiences [52].
The catalytic subunit of PCR2 is the enhancer of zeste homolog 2 (EZH2) and has been found to be mutated and overexpressed in several subtypes of PTCL. Mutations in EZH2 have been shown to produce transcriptional silencing, leading to lymphomagenesis; inhibition of EZH2 of PTCL lines has induced marked cell growth arrest via marked upregulation of genes involved in cell cycle [10, 51, 53] In primary cutaneous ALCL (C‐ALCL), EZH2 mutations repress antitumor immunity via suppression of the C‐X‐C motif chemokine ligand 10/receptor 3 axis, which is important in T‐cell migration in inflammatory conditions, including PTCL [53].
Another example of an epigenetic lesion that may contribute to PTCL pathogenesis is KMT2D (also known as MLL2 ) [3, 51]. KMT2D encodes histone H3K4 methyltransferase. Mutations in this gene have been seen in 42% of PTCL in general, with approximately 25 and 36% of patients with the AITL and PTCL‐NOS subtypes carrying the mutation [54]. Figure 3.1shows common epigenetic mutations, targets, and the drugs that can modulate this biology ( Figure 3.2).
In addition to mutations in genes directly effecting epigenetic processes, TCLs commonly have mutations in pathways that can directly or indirectly affect this biology. For example, a gene found to be commonly mutated in PTCL is RHOA . While RHOA is not thought to be involved in maintaining the PTCL epigenome, it does belong to the Rho family of small GTPases, a group of Ras‐like proteins involved in intracellular signaling. Gain of function mutations in RHOA are seen in 50–70% of AITL compared with 15% in ATLL (with its own unique distribution of mutations) and are associated with cell proliferation and invasiveness [8, 35, 43]. In patients with AITL, 70% of cases are associated with a specific mutation in RHOA G17V, which appears to be correlate with paraneoplastic autoimmunity and lymphopenia [8, 55]. Preclinical models have shown that mice with a TET2 mutation as the initiating event and RHOA G17V mutation as the second hit develop TCLs with histologic and immunophenotypic features of AITL [35, 55–57]. Murine lymphoma models have shown that RHOA G17V leads to a reduced threshold for T‐cell receptor activation and can augment the PI3K‐AKT‐mTOR signaling signature [56]. In these murine models, treatment with duvelisib, the PI3K inhibitor, can produce significant reductions in tumor burden, while treatment with everolimus improved survival. This mouse model confirms that these mutations are pathogenic and provide rationale for interrogation of drugs targeting the epigenome as a therapeutic target in PTCL.
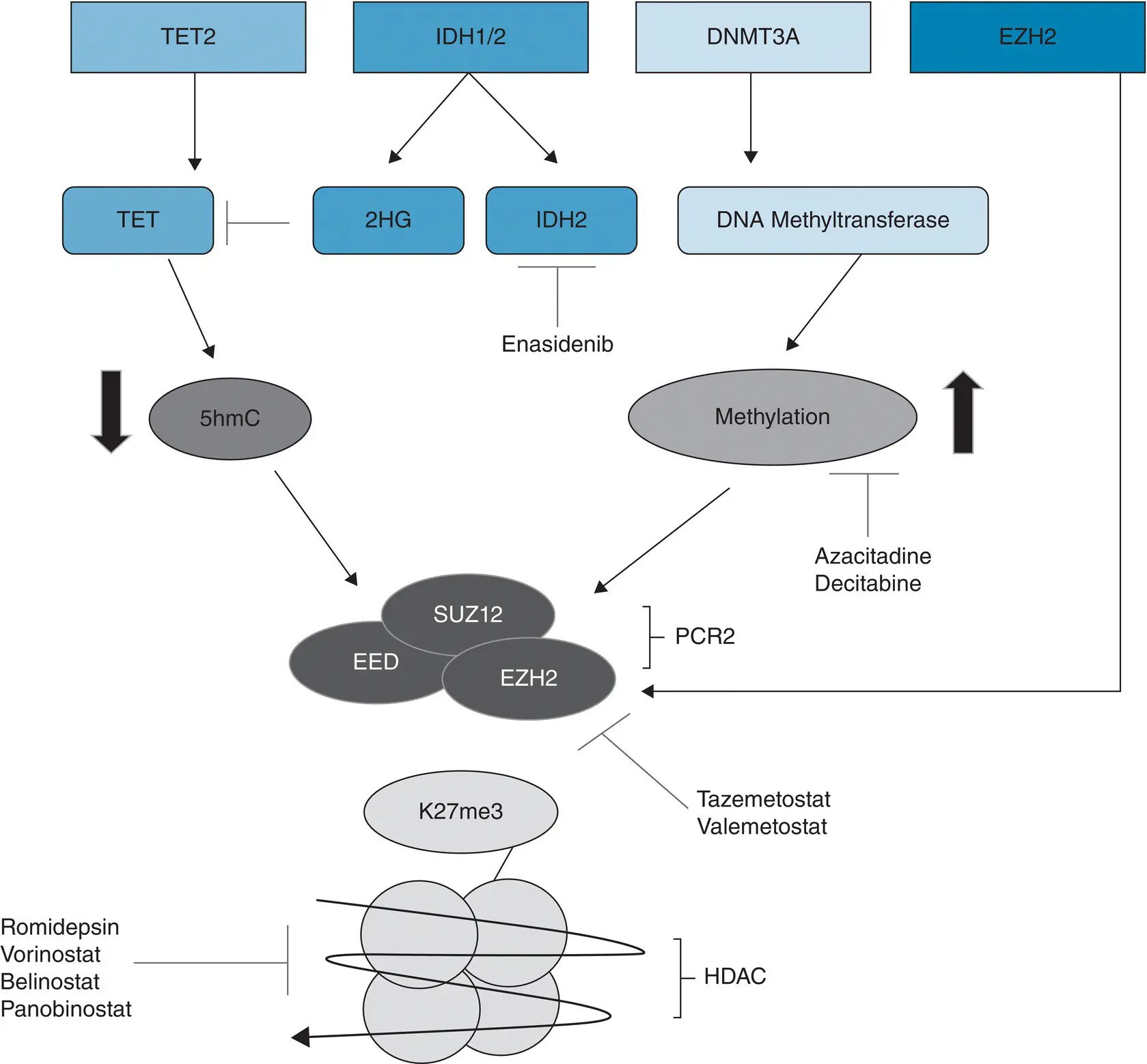
Figure 3.1 Common epigenetic targets in peripheral T‐cell lymphoma. TET = ten eleven translocation protein, 2HG = 2‐hydroxyglutarate, IDH2 = isocitrate dehydrogenase 2, 5hmC = 5‐hydroxymethylcytosine, EED = embryonic ectoderm development, SUZ12 = suppressor of zeste 12, EZH2 = enhancer of zeste 2, PCR2 = polycomb repressive complex 2, K27me3 = trimethylation at lysine 27 of histone 3 (aka H3K27me3), HDAC = histone deacetylase.
Читать дальше













