Variation in microenvironment may have prognostic relevance, reinforcing its role in lymphomagenesis. Tissue infiltration of CD163‐positive macrophages has been shown to associate with worse prognosis in patients with AITL, suggesting their importance in AITL [67]. A depletion of Treg cells and an expansion of CD163 macrophages together with an accumulation of Th17 cells reported in AITL tissues could contribute to the proinflammatory and immunosuppressive microenvironment in AITL [68]. Gene expression studies identified molecular signatures associated with outcome [69, 70]. For example, The B‐cell‐associated signatures predicted favorable outcome, whereas monocytic, cytotoxic (associated with CD8 + T cells) and p53‐induced target gene signatures were associated with poor outcome [70].
Genetic Alterations in the Angioimmunoblastic T‐cell Lymphoma Microenvironment
Recent genetic studies at the single‐cell level have shed a new light onto the bystander cells in AITL. Since founding mutations in epigenetic modifiers have been found to occur in a hematopoietic CD34+ precursor or stem cell before lineage commitment [17, 31], TET2 and DNMT3A mutations can be detected not only in neoplastic T cells, but also in B cells isolated from AITL biopsies [71, 72]. Variant allele frequencies of TET2 and DNMT3A are usually higher than those of T‐cell restricted RHOA or IDH2 variants [14], indicating that epigenetic deregulation more widely affects the different cellular components of the tumors. Moreover, the B blasts, in addition to those infected with EBV, can show a restricted repertoire of hypermutated IG genes with destructive mutations [73], and may harbor additional somatic mutations, notably in NOTCH1 [72]. It is likely that this could hold true for reactive CD8+ T cells often abundant in AITL tissues and for mononucleate cells of myeloid lineage. In this respect, it is noteworthy that co‐occurrence of myeloproliferative disorders like acute myeloid or chronic myelomonocytic leukemia and AITL have been reported in some patients [74–76]. Both diseases may derive from a common ancestral progenitor harboring TET2 and/or DNMT3A mutations and/or high‐risk clonal hematopoiesis, with subsequently acquisition of distinct mutations initiating the divergent development of AITL and myeloid disease [75]. Altogether, these findings indicate that several components of the microenvironment can be genetically altered in AITL, and it is tempting to speculate that genetically altered bystander cells could also contribute to AITL pathogenesis ( Figure 2.2). The role of EBV in this disease remains debated. It infects a variable number of large B blasts in around 80% of cases, with a latency type 2, but the question whether EBV has any oncogenic role or is present as a passenger, reflecting the immune defect observed in AITL, remains open. Indeed, transforming growth factor beta and IL10 produced by Tfh cells are known to suppress T‐cell responses by inhibiting the proliferation and function of conventional CD4 Th1 cells, and may play a role in the expansion of EBV‐positive B cells.
Specific Microenvironment Components Present in Other Primary Cutaneous T‐cell Lymphoma Entities
A microenvironment component is present in all PTCL entities. Here, we review the data relative to distinct cellular components.
A high content in histiocytes is present in some PTCL entities. For example in the lymphohistiocytic variant of ALCL ALK‐positive , which occurs exclusively in children and young adults, the gene expression signature is largely contributed by the histiocytic component and, in comparison to the classical disease the patients have a more disseminated disease with tendency to a leukemic picture and a worse prognosis with a high risk of failure [77]. Conversely, the lymphoepithelioid variant of PTCL, NOS tends to be associated with an overall better prognosis than other PTCL‐NOS [78, 79]. In PTCL, NOS rich in histiocytes, the molecular signature related to inflammatory response (chemokines, cathepsins, major histocompatibility complex (MHC) molecules, genes involved in the interferon response pathway) and to the monocyte–macrophage background appears to be inversely related to a proliferation signature and associated with an adverse prognosis [80, 81].
Among non‐Hodgkin lymphomas, PTCLs demonstrate the highest microvessel density [82]. Increased microvessel density has also been shown in cutaneous biopsies involved by mycosis fungoides in comparison with inflammatory skin conditions [83]. VEGF overexpression in PTCL, NOS has been associated with a poor outcome [84]. VEGF may result in local neovascular transformation (angiogenesis) and recruitment of circulating progenitors derived from the bone marrow (vasculogenesis) [85]. However, in contrast to AITL, it is unclear which cell type(s) release VEGF and whether VEGF receptors are present and functional on the lymphoma cells in PTCL, NOS. In models of NPM‐ALK‐driven oncogenesis, STAT3 induces VEGF expression by the lymphoma cells. MicroRNA‐135b is another mediator of NPM‐ALK‐mediated angiogenesis [86]. Elevated serum VEGF levels in patients with non‐Hodgkin lymphoma have been reported to be associated with a poor outcome [87]. Attempts to improve the outcome of patients with PTCL by adding bevacizumab have, however, proven ineffective [88].
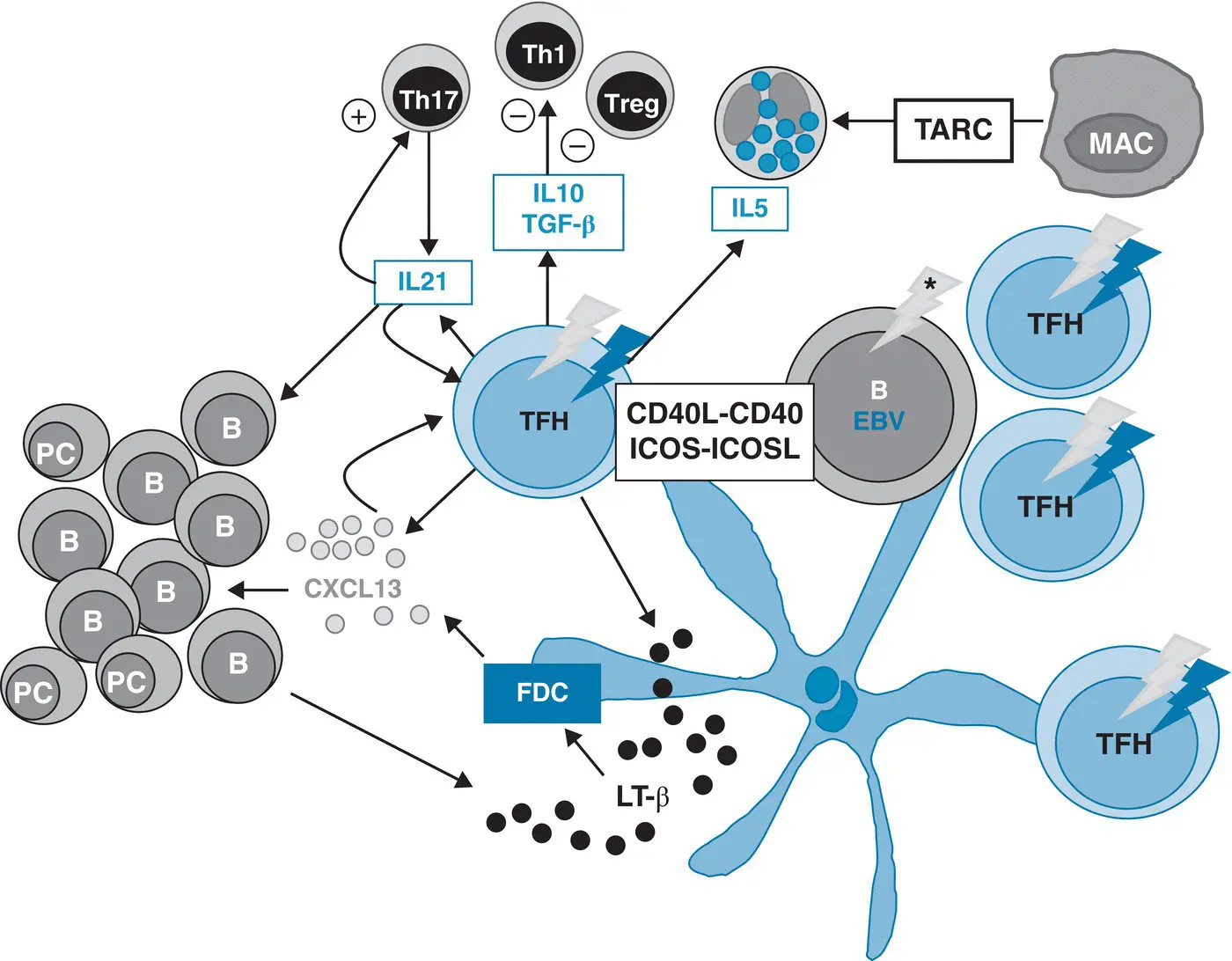
Figure 2.2 Neoplastic T follicular helper cells and their microenvironment in angioimmunoblastic T‐cell lymphoma. B, B cell; EBV, Epstein–Barr virus; FDC, follicular dendritic cell; IL, interleukin; LT‐β, lymphotoxin‐beta; MAC, macrophage; PC, plasma cell; TFH, T follicular helper cells; TGF, transforming growth factor; Th1, T lymphocyte 1; Treg, T regulatory cells (gray arrows: epigenetic mutations, which may occur early and may be detected in neoplastic cells and other reactive cells including B cells; asterisk: additional mutations or somatic hypermutations; red arrows: T‐cell‐specific mutations like RHOA G17V or others.
Source: Adapted from de Leval and Gaulard [63].
Blood and tissue eosinophilia may be seen in various PTCLs as a result of non‐clonal expansion of normal eosinophils mediated by eosinophilopoietic growth factors, such as IL3, granulocyte‐macrophage colony stimulating factor and IL5 normally produced by activated T cells (for review see Roufosse F et al. [89]). Other factors promoting eosinophil chemotaxis include RANTES (regulated on activation normal T cell expressed and secreted)/CCL5 and eotaxins 1–3 (CCL11, CCL24, CCL26). RANTES also exerts chemoattractant activity for T lymphocytes, monocytes, and basophils, and is produced by a variety of cell types, including T cells, fibroblasts, and epithelial cells. Eotaxins signal through CCR3 receptor, which is expressed at high levels on eosinophils and Th2 cells. Cellular sources of eotaxin include fibroblasts, endothelial cells, eosinophils, and lymphocytes.
PTCLs most commonly associated with eosinophilia include primary CTCL, AITL, and ATLL. Approximately 15–20% of patients with mycosis fungoides and up to 75% of those with Sézary syndrome develop blood eosinophilia and show numerous eosinophils within cutaneous lymphoma infiltrates. Eosinophilia in mycosis fungoides and Sézary syndrome, is related to the Th2 nature of the lymphoma cells, which produce IL4, IL5, and IL13. The recruitment of eosinophils to the skin is mediated by chemoattractants produced by the lymphoma cells but also possibly by other cell types. Indeed, IL4‐producing lymphoma cells induce increased expression of eotaxin‐3/CCL26 by keratinocytes, endothelial cells, and fibroblasts [89]. In cutaneous ALCL as well lymphomatoid papulosis (type A), a reactive inflammatory infiltrate may be found within the tumor, and eosinophils may be detected in a substantial proportion of patients occasionally representing the predominant cell type. CD30 +tumor cells isolated from skin biopsies with cutaneous ALCL have been shown to coexpress CCR3 and IL4, and eotaxin in conjunction with surrounding cells, indicating that they contribute to eosinophilic infiltrates [90].
Читать дальше













