3.2.3 Chain Transfer Reaction
Chain transfer reaction then occurs in which an active center is transferred from a growing oligomer molecule to another molecule.
3.2.4 Termination Reaction
Termination reaction involves the growing polymer sites reacting together. Free radical polymerization can also be terminated or retarded by the presence of atmospheric oxygen. Several techniques are used to prevent this termination or retardation of the free radical cure, especially at the interface between the coating and ambient air. We will discuss in a later section methods and techniques to minimize the retardation or termination of the free radical cure by oxygen [2].
The most significant method of cure for acrylated oligomers and monomers is through the use of UV light and a PI. The PI acts as the catalyst to free-radically cure the system instantaneously. An example of a PI is hydroxypropiophenone shown in Figure 3.2.
The cleavage reaction of 2-hydroxy-2-methyl-1-phenyl-propan-1-one when it is photolyzed is shown in Figure 3.2. This proposed cleavage goes through a very short duration triplet state and decomposes by α-splitting to give a benzoyl radical and a 2-hydroxy-2-propyl radical which cross-link the system [2, 3].
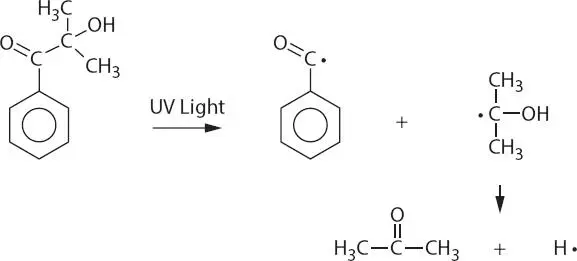
Figure 3.2 Photoinitiation: UV light hits the 2-hydroxy-2-methyl-1-phenyl-propan-1-one resulting in a cleavage reaction that goes through a short duration triplet state and then decomposes by splitting to give a benzoyl radical and a 2-hydroxy-2-propyl radical which cross-link the system.
3.3 UV Cure Light Sources: Gallium-Doped Low-Wattage Long Wavelength Fluorescent (FL) Bulbs and Light Emitting Diodes (LEDs)
3.3.1 UV Light Spectrum
To activate the PI a UV light source is needed that is tailored to the proper wavelength. To understand this better we need to review the wavelengths of UV light sources that are available commercially.
As one can see in Figure 3.3, UV light sources are available in the UV-A (long wavelength: starts at 365 nm), UV-B (midrange wavelength: starts at 302 nm) and UV-C (short wavelength: starts at 254nm).
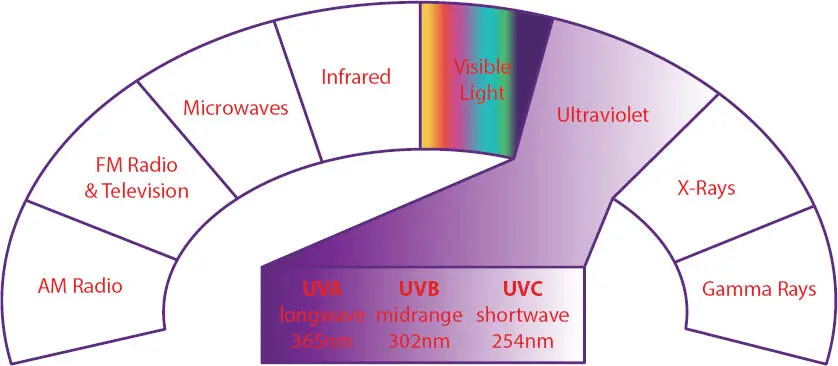
Figure 3.3 Electromagnetic spectrum. UV-C (shortwave) starts at 254 nm, UV-B (midrange) starts at 302 nm and UV-A (longwave) starts at 365 nm. UV cure light sources used in the UV nail gel area use the UV-A source.
3.3.2 Matching the PI with the UV Light Source and Pigments Absorption/Transmission
It is important when selecting a PI that every effort is made to match the PI to the wavelength of the UV light source to obtain maximum crosslinking of the coating. In pigmented coatings this becomes even more important due to the absorption of UV light by most pigments. As can be seen in Figure 3.4a rutile version of titanium dioxide ((TiO 2)) has the following absorption/transmission spectrum.
This absorption/transmission spectrum is critical when using certain PIs. In fact, UV light sources that are in the UV-B and UV-C ranges as shown in Figure 3.3cannot fully activate the PIs through to the bottom of the applied coating. The formulator then needs to find a light source that operates in the UV-A wavelength since the rutile (TiO 2) will block the absorption of the UV light.
Figure 3.5shows the proper source of UV-A wavelength that operates above 365 nm using gallium to spectrally shift a traditional low-wattage long-wavelength fluorescent (FL) bulb to be above the absorbance of the rutile (TiO 2).
It is important to utilize PIs that are not blocked by the rutile (TiO 2) as can be seen in Figure 3.6. Typically, PIs (PIs a, b & c shown in Figure 3.6) that are used in unpigmented coatings will not work with pigmented systems since the UV light is not able to penetrate the coating and will result in a cured upper surface and an uncured lower surface. However, to resolve this issue PIs that operate in the 380 nm and above cut-off will activate and fully cure the coating all the way to the substrate as shown in Figure 3.6(PI-d).
UV light sources and their intensities also play a significant role in curing and through-curing the pigmented coating. As can be seen in Figure 3.7pendulum hardness values vary greatly depending on the UV light source utilized.
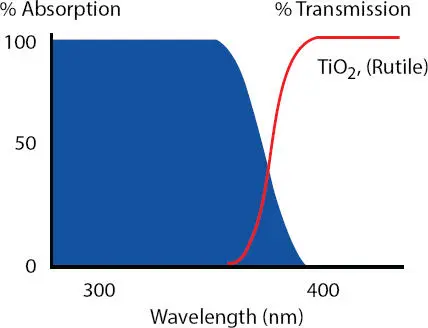
Figure 3.4 Absorption/transmission spectrum of rutile ((TiO 2)) that is important to consider when attempting to cure pigmented UV nail gel formulations.
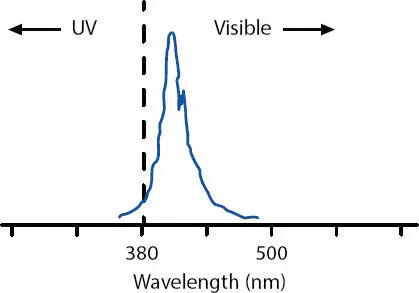
Figure 3.5 Emission spectrum of gallium-doped (Phillips-60 W TL03) low-wattage long-wave fluorescent bulb. Gallium is used to give a spectral shift upwards so it matches the PI.
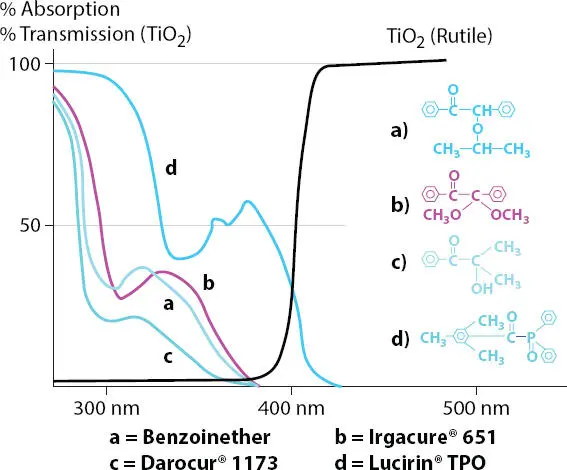
Figure 3.6 Absorption curves for photoinitiators used. a=Benzoinether absorbs in the 280 to 360 nm range, b=Irgacure 651 absorbs in the 280 to 360 nm range, c=Darocur 1173 absorbs in the 280 to 360 nm range and is blocked by the rutile (TiO 2). d= Lucirin TPO absorbs above 365 nm to allow proper through-cure of the coating due to high pigment loading by rutile (TiO 2).
The ability to cure coatings that contain up to 30% rutile ((TiO 2)) has an impact on through-cure. The higher the pigmentation the less the chance for through-cure. In addition, a thicker pigmented coating also results in no through-cure. Using the same UV cure formulation, researchers have shown that by just increasing the energy density one can get better through-cure as shown in Figure 3.7(a & b) using UV arc lamps at 200W and 300 W. By utilizing the addition of TL03 UV Lamp (Gallium-doped low wattage long wavelength fluorescent; Phillips 60 W) one enhances the deep penetration of the UV light into the coating which results in better through-cure and hardness development. Even better performance results are shown in Figure 3.7(E-Gallium Doped & F-Iron Doped) and the use of high performance (300 W and 600 W) UV light sources results in the best through-cure and hardness development [4].
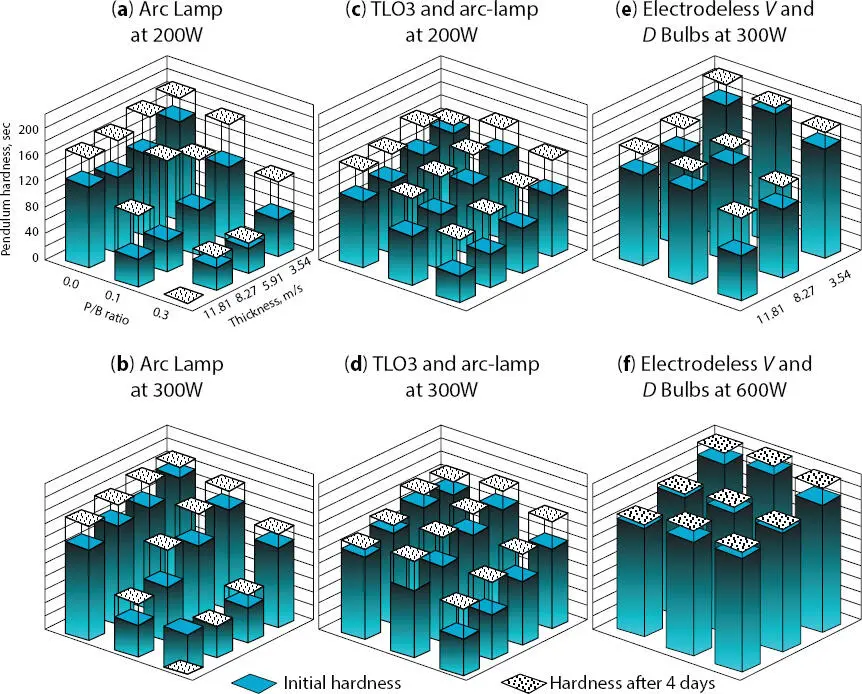
Figure 3.7 UV through-cure of various rutile ((TiO 2)) formulations; Pendulum Hardness Values, sec vs. P/B (pigment to binder) ratio vs. thickness, µm. These formulations were compared against (a) UV Arc @ 200W & (b) 300W, (c) TL03 (GA-FL; 60W) @ 200W & (d) TL03(GA-FL; 60W) @ 300W, (e) Electrodeless V (GA) & D (Fe) @ 300W and (f) Electrodeless V (GA) & D (Fe) @ 600W UV light sources.
In the UV nail gel market the first UV light sources were gallium-doped low-wattage long wavelength fluorescent bulbs (GA-FL) that are close to Figure 3.7(c & d) UV wavelength and energy levels except for the use of the UV arc lamp bulbs. Early work with these GA-FL bulbs allowed the formulator to cure oligomeric chemistries in about 3 minutes.
Читать дальше


















