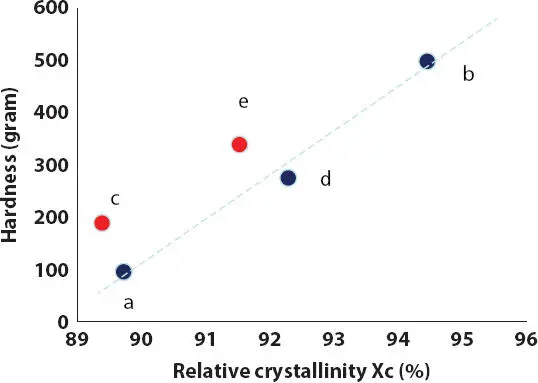
Figure 2.10 Effect of the % relative crystallinity X c(from wax melting) on the hardness of the lipsticks containing 15% PE wax in HPIB oils.
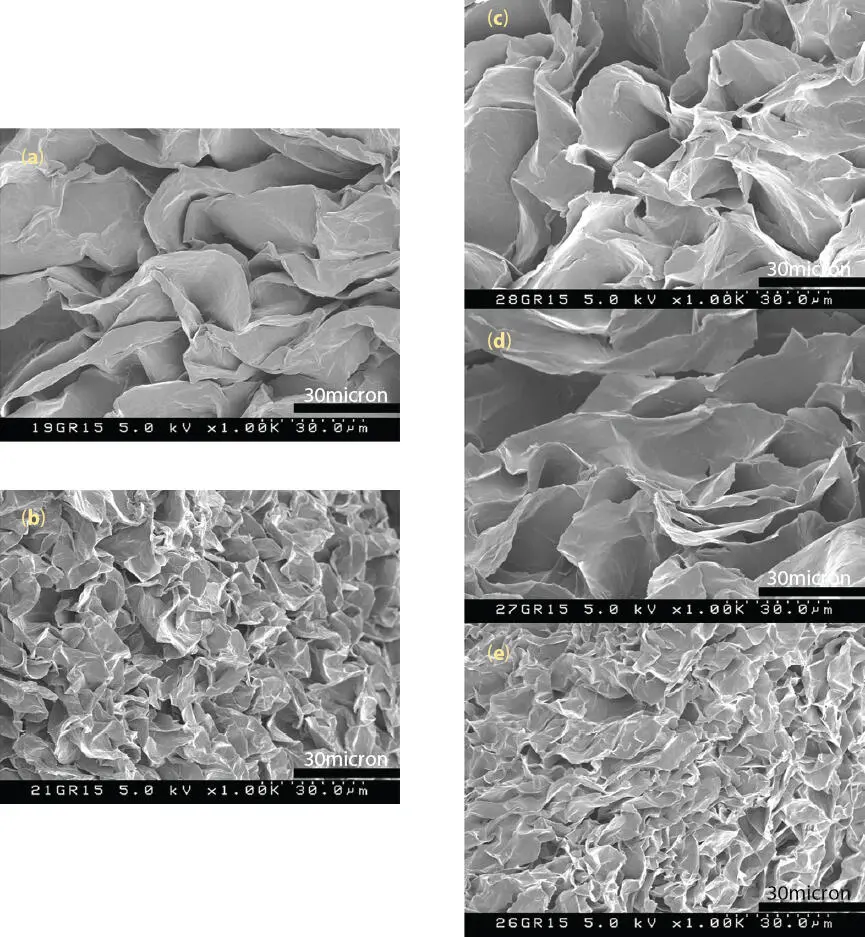
Figure 2.11 SEM micrographs of PE wax-oil lipsticks at fixed 15% PE wax in hydrogenated polyisobutene (HPIB) with oil viscosity (a) ɳ HPIB= 15 mPa.s, (b) ɳ HPIB= 89,960 mPa.s, and with oil blend viscosity (c) ɳ HPIB blend= 72 mPa.s, (d) ɳ HPIB blend= 900 mPa.s and (e) ɳ HPIB blend= 9,200 mPa.s.
From these results, the viscosity of the oil-wax system during cooling has a strong effect on the crystal nucleation and growth. When the temperature is reduced during the cooling process, the viscosity of oil-wax solution increases and crystals start to nucleate in oil and aggregate to form a gel network. In a high viscosity solution, the wax solution becomes gel faster during the cooling process, and the nucleation and growth of crystals will slow down, forming smaller wax crystals compared to the wax crystallized in a lower viscosity solution. Depending on the viscosity of the oil-wax gel at the gelling temperature, the aggregation of crystals would influence the strength of wax network and the final oil-wax lipstick structure. Therefore, the oil viscosity has a strong impact on the crystallization of PE wax, resulting in different crystal sizes, shapes and densities as observed from SEM in Figure 2.11. The dependence of the hardness of the oil-wax system on the oil viscosity was also observed and discussed by Abidh et al . [34] or by Dassanayake and coworkers when they studied the kinetics of crystallization of rice bran wax (RBW) in vegetable oils [38].
2.5.2 Factors Affecting Lipstick Structure: Oil Polarity
To test the effect of polarity on the crystallization of PE wax in oils, a series of ester oils with various viscosities and polarities were selected and are shown in Table 2.6. The oil polarity was measured in terms of relative permittivity (RP) for ester oils with various chemical structures. For comparison, hydrogenated polyisobutene (HPIB) a non-polar oil with low polarity was included in the data analysis. Oils with high RP are more polar than oils with low RP. For example, tri-decyl trimellitate with RP of 4.11 is more polar than isononyl isononanoate with RP of 3.29.
At a fixed 15% PE wax, the oil-wax lipstick hardness increases with increasing oil polarity (relative permittivity) ( Figure 2.12). However, it is observed that the hardness of PE wax in HPIB with low RP of 2.25 and in polyglycerin-2-tri-isostearate with high RP of 3.35 does not follow the trend. This phenomenon might be due to the contribution of the high viscosity from these two oils, as both HPIB and polyglycerin-2-tri-isostearate oils have viscosities of 8.9 x10 4mPa.s and 2.4x10 4mPa.s, respectively.
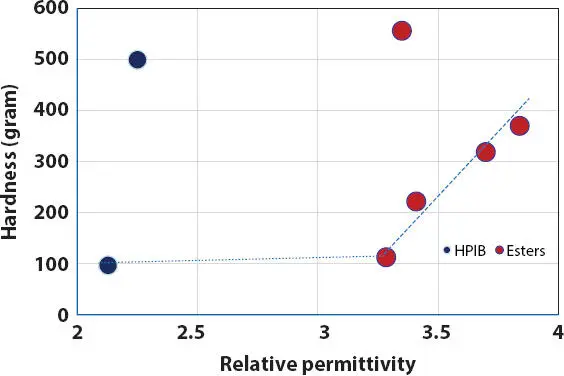
Figure 2.12 Effect of oil relative permittivity on the hardness of wax-oil lipstick containing 15% PE wax.
DSC thermograms of crystallization and melting of 15% PE wax in various polar oils are shown in Figure 2.13. It is observed that by increasing RP or polarity of oils, both the starting crystallization T c,iand final melting temperatures T m,fof PE wax do not increase linearly as expected. This might be due to the contribution of the oil chemical structure besides the oil polarity. The melting peak temperature T mof wax crystals and the relative crystallinity X cdetermined from the wax melting process are observed to increase with increasing oil polarity as shown in Figures 2.14- 2.15. However, it is not true for high viscosity ester polyglycerin-2-tri-isostearate as discussed above, which has the highest relative crystallinity and high T meven its RP is lower compared to other ester oils. In addition, the wax in low polarity HPIB has similar crystallinity to wax in high polarity ester.
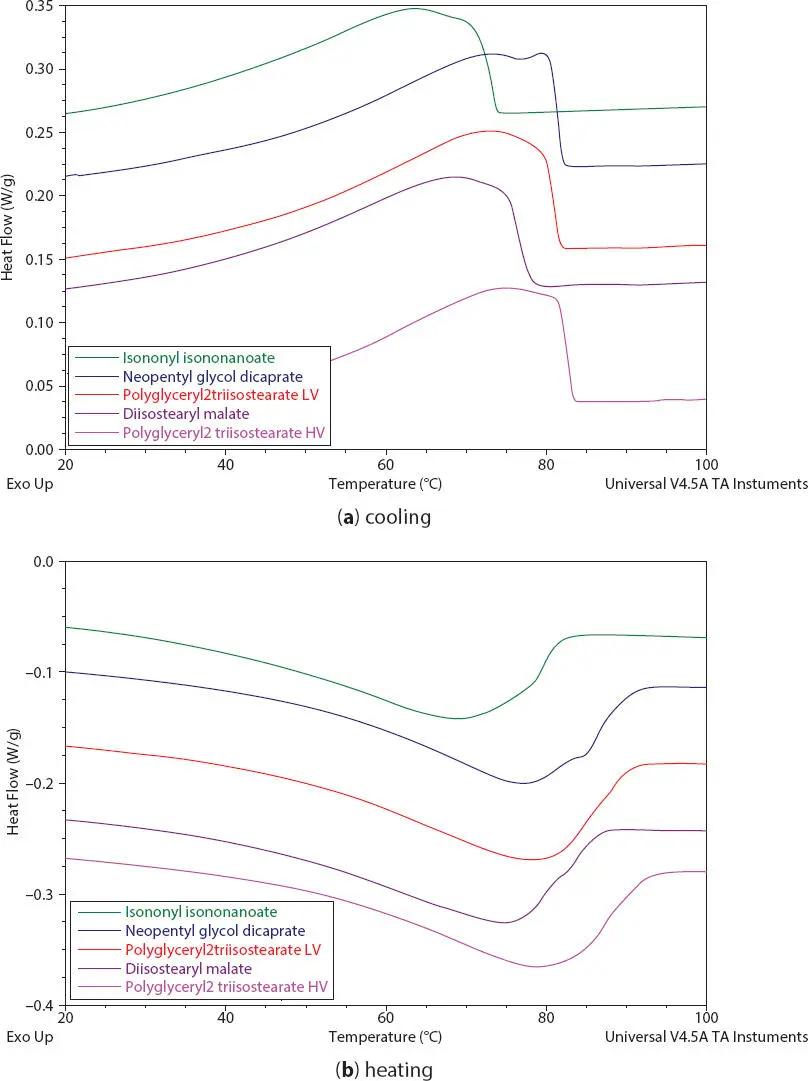
Figure 2.13 DSC thermograms of 15% PE wax in polar oils (esters) during (a) cooling and (b) heating processes.
Therefore, the oil viscosity might play a more important role in the crystallization of PE wax than the oil polarity in the simple oil-wax lipstick. As a result, the T mand hardness of the wax-oil lipsticks are better correlated with viscosity of oils than with oil polarity as shown in Figures 2.16- 2.17. In addition, the hardness of lipstick structure also corresponds with the relative crystallinity X cdetermined from the melting of the PE wax in ester oils ( Figure 2.18).
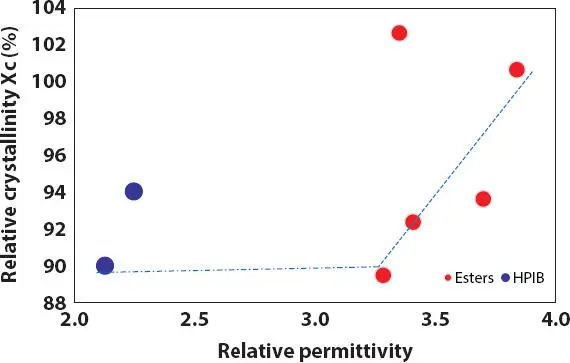
Figure 2.14 Effect of relative permittivity (polarity) on the relative crystallinity X c(%) of lipstick containing 15% PE wax in oils (red circles = esters, blue circles = HPIB).
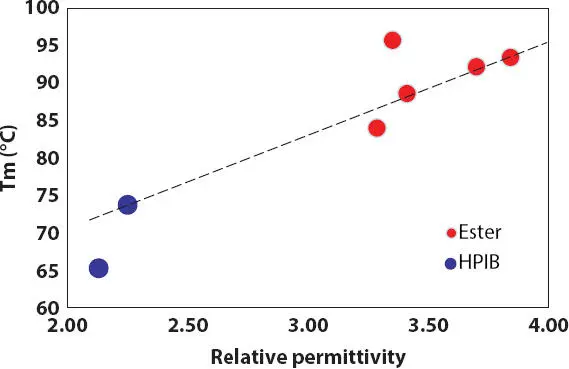
Figure 2.15 Effect of relative permittivity (polarity) on the wax melting peak T mof lipstick containing 15% PE wax in oils (red circles = esters, blue circles = HPIB).
The morphology of the wax crystals from the polar oil-wax lipsticks studied by SEM is shown in Figure 2.19. The oil polarity has some effect on the crystal structure of PE wax, resulting in more compact structure when oil polarity increases. In a low viscosity and low polarity isononanoate, the wax crystal is the largest as observed for PE wax in the low viscosity HPIB oil from SEM in Figure 2.11. The wax crystals of sample ein Figure 2.19have the smallest crystal size with high crystal density, contributing to the hardest lipstick structure even if the oil has lower polarity but it has the highest viscosity.
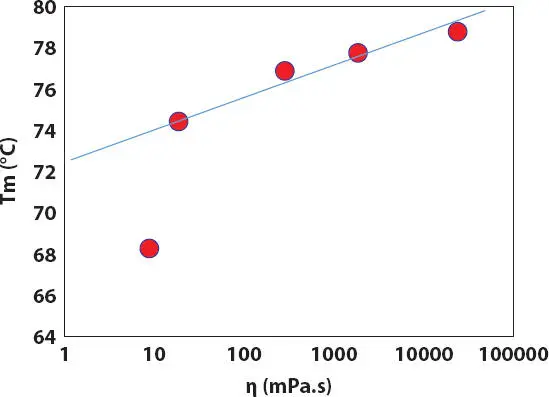
Figure 2.16 Effect of oil viscosity on the melting temperature Tm of PE wax crystals in ester oils during heating.
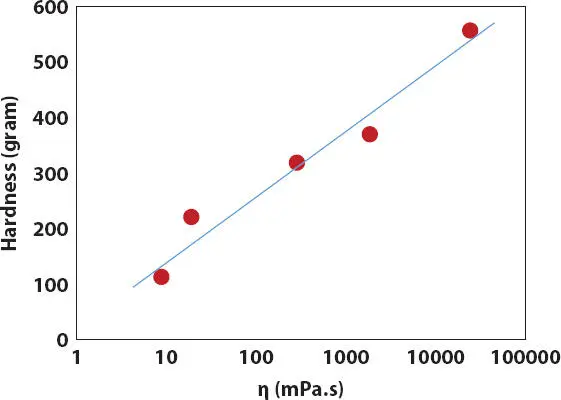
Figure 2.17 Effect of oil viscosity on the hardness of lipstick structure containing 15% PE wax in ester oil.
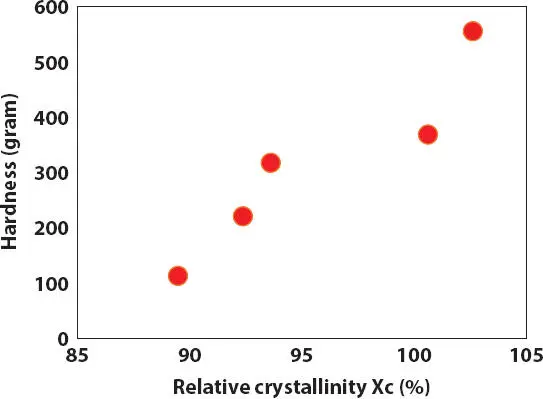
Figure 2.18 Effect of the relative crystallinity X c(%) obtained from crystal melting on the hardness of lipstick containing 15% PE wax in ester oils.
However, the hardness of lipstick structure was found to correlate better with oil viscosity than with oil polarity. For a better understanding of the effect of oil polarity on the wax crystal structures, ester oils with similar viscosity but different polarity should be used.
Читать дальше





















