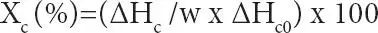2.3.3 Factors Affecting Oil-Wax Structures: Oil Viscosity
De Clermont-Gallerande et al . [33] reported the roles of oil viscosity and wax amount in the lipstick formulation on the mechanical properties of the lipsticks. By varying the oil viscosity or wax amount or wax type, they could control the hardness and the deposit of the lipstick which affect the sensorial perception. For example, the use of a highly viscous oil in the synthetic wax - polyethylene - polypropylene/ethylene copolymer (T m= 82 0C) increases both the breaking resistance and the thickness of lipstick compared to low viscosity oil. Using DSC, Abidh and coworkers also confirmed the strong impact of the oil viscosity on the wax crystal network which influenced the mechanical and sensorial properties of model lipsticks in a recent publication [34].
2.3.4 Factors Affecting Oil-Wax Structures: Cooling Rate
The cooling rate will affect the nucleation, crystal growth and solidification of oil-wax systems. With high cooling rate, wax crystals are smaller and have high crystal density than at low cooling. At low cooling rate, the crystal growth rate is dominant over nucleation, resulting into larger crystals.
The crystal growth from rice bran wax in hexane (non-polar oil) was investigated by using a laser diffraction technique. Ghosh and Bandyopadhyay [31] found that a high cooling rate and low temperature induced formation of a large number of small nuclei and maximal crystal size was obtained in the temperature range of 10°C-15°C.
Hwang and coworkers showed that with an increase of cooling rate from 0.1°C/min to 4°C/min, the crystal sizes of 4% sunflower wax in soybean oil reduced and the firmness of the oil-wax gels increased due to high density of small crystal formation [29].
Therefore, the hardness or firmness of oil-wax system can be modulated by cooling rate at a fixed wax concentration. A fast cooling rate will have a large impact on the hardness of the oil-wax system when a high concentration of wax is used.
2.4 Study On Model Oil-Wax System Containing Polyethylene Wax
To modulate the hardness of the solid lipstick, this study investigates the factors that might affect the hardness of simple oil-wax systems such as viscosity and polarity of various cosmetic oils with polyethylene wax. The crystallization and structures of the low molecular weight PE wax in such systems were investigated by DSC and SEM. Then the correlation between the hardness of the lipstick and its deposit amount on the bioskin is discussed.
Polyethylene wax with low Mw of 500 and T m= 83°C- 90°C was used as the only wax in the study of lipsticks. The common esters and hydrocarbon oils used in lipstick formulations were selected with various viscosities and polarities (in terms of permittivity) ( Table 2.6). PE waxes in a single oil or in binary oil blends of hydrogenated polyisobutene (HPIB) with low and high viscosities (ɳ=14.8 mPa.s and 89960 mPa.s) were prepared by melting the waxes in oils at high temperature.
Table 2.6 Some selective non-polar HPIB and polar oils with low and high viscosities.
| Oils |
Viscosity η (mPa.s) |
Relative permittivity |
| Hydrogenated polyisobutene |
15 |
2.1 |
| Hydrogenated polyisobutene |
89960 |
2.3 |
| Isononyl isononanoate |
9 |
3.29 |
| Neopentyl glycol dicaprate |
19 |
3.41 |
| Polyglyceryl-2 tri-isostearate |
290 |
3.7 |
| Diisostearyl malate |
1860 |
3.84 |
| Polyglyceryl-2 tri-isostearate |
24200 |
3.35 |
2.4.2 Measurements
2.4.2.1 Oil Viscosity
A Haake rheometer model RS75 with a cone diameter of 60 mm and 2° angle was used to measure the viscosity of single oils and their mixtures at room temperature. The flow curve was obtained as a function of stress from 0.1 Pa to 1000 Pa.
2.4.2.2 Oil Polarity by Relative Permittivity
Oil polarity as relative permittivity can be measured by an LCR meter by Agilent Technologies [35]. Permittivity ε is a measure of the polarizability of a solvent (responsiveness of a localized charge distribution (dipole) to an external electric field), a property which is related to the polarity of the solvent molecules.
Permittivity is defined as the ratio D/E where D is the electric flux density and E is the electric field strength. The permittivity is specified in Farads per meter (F/m). It can also be defined as a dimensionless relative permittivity, or dielectric constant, normalized to the absolute vacuum permittivity ε 0= 8.85419 x10 -12F/m.
2.4.2.3 Hardness of Lipsticks
A simple lipstick containing polyethylene wax and oil was prepared by heating the mixture above 95°C until it melted completely. Then the hot mixture was poured into a lipstick mold and cooled down in a refrigerator for 20 min. The lipstick was molded and placed in a 12mm diameter bullet shape container. The lipstick was then stored at 20°C for 24 hours before measuring its hardness.
The hardness of the lipstick samples was measured using a DFGS2 dynamometer (Indelco-Chatillon). The hardness corresponds to the maximal shear force exerted by a rigid tungsten wire of diameter 250 μm, advancing at a speed of 100 mm/min.
The technique above is normally described as the “butter- cutting wire” method. The hardness value from this method is expressed in grams as the shear force required to cut a lipstick under the above conditions.
2.4.2.4 Amount and Thickness of Lipstick Deposit on Bioskin
Simple lipstick containing a single oil with polyethene wax was applied twice on a flat-type Bioskin from Beaulax Co., Ltd, Japan. The application amount of lipstick on the Bioskin was determined from the weight difference after and before application. Then, the thickness of lipstick deposit (film thickness) can be calculated from the values of application area, weight of deposit and density of lipstick.
2.4.2.5 Wax Crystallization Study
DSC was used to study the crystallization and melting behaviors of wax and wax-oil systems. The samples were heated from -30 to 140°C at a rate of 5°C/ min. After holding at 140°C for 5min, the samples were allowed to crystallize upon cooling at 5°C/min from the melt of 140°C to - 30°C. The samples were heated again from -30°C to 140°C at the same rate as cooling. Figure 2.3shows the cooling and heating cycles for an oil-wax gel system. The parameters such as the crystallization temperature, T c, melting temperature T mand the enthalpies of crystallization ΔH c(J/g) and melting ΔH m(J/g) of the wax – oil systems were obtained from DSC thermograms. In addition, the starting crystallization temperature T c,iand final melting temperature Tm,f are used in the analysis. The relative crystallinity of the samples during cooling or heating process, X c(%), can be calculated using enthalpy of crystallization or enthalpy of melting value of 100 % crystalline polyethylene wax (PE).
The relative crystallinity X c(%) from cooling or from melting is calculated via either equation:
(2.1) 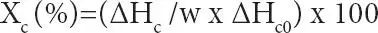
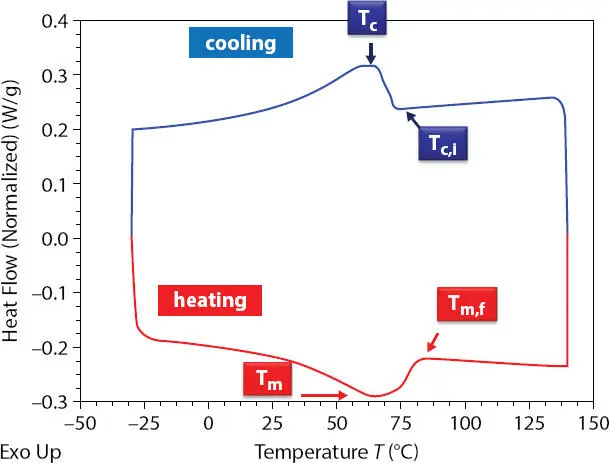
Figure 2.3 DSC thermogram of an oil-wax gel system.
Читать дальше