In the case of white Champagne base wines, it has proved possible to recycle the tartrate four times, with almost constant treatment effectiveness ( Table 1.17). The continued effectiveness of the treatment, even when the tartrate has been recycled four times, has been explained (Maujean et al ., 1986). It was shown that the smallest particle size after treatment (<50 μm) was larger than the initial size in the commercial product.
TABLE 1.17Changes in the Physicochemical Parameters of Cold‐Stabilized Wine When the Contact Tartrate Was Recycled (Maujean et al., 1986)
| Number of times used |
K +(mg/l) |
Total acidity (g/l H 2SO 4) |
Tartaric acid (g/l H 2SO 4) |
pH |
pC × 10 5 |
| 1 |
315 |
4.93 |
1.59 |
3.11 |
6.83 |
| 2 |
325 |
4.92 |
1.54 |
3.12 |
6.88 |
| 3 |
320 |
4.90 |
1.59 |
3.11 |
6.84 |
| 4 |
300 |
4.98 |
1.83 |
3.09 |
7.35 |
| 5 |
320 |
4.94 |
1.55 |
3.08 |
6.57 |
Of course, recycling is not possible when red wines are treated, as the crystals become coated with phenols and coloring matter and rapidly lose their effectiveness.
1.7.4 Rapid Cold Stabilization: Dynamic Continuous Contact Process
Unlike the preceding “batch” technology, the process described in Figure 1.17is a continuous bitartrate stabilization process, where the length of time the crystals are in contact with the wine, i.e. the treatment time, is defined by the throughput in relation to the volume of the crystallizer. Thus, for example, if the throughput is 60 hl/h and the volume of the crystallizer is 90 hl, the average time the wine spends in the system is 1 hour 30 minutes.
This emphasizes the need for a method of monitoring effectiveness with a very short response time. There is, of course, a system for recycling wine through the crystallizer if the treatment is insufficiently effective, but the results must be determined very rapidly, as the energy required to treat these quantities of wine is expensive, and unnecessary extra treatment will by no means improve quality.
Continuous treatment is understandably more demanding than the other processes, because it requires close monitoring, but it is also more efficient. For example, the particle size of the contact tartrate and the level in the crystallizer must be monitored by sampling after a few hours, using the drain system.
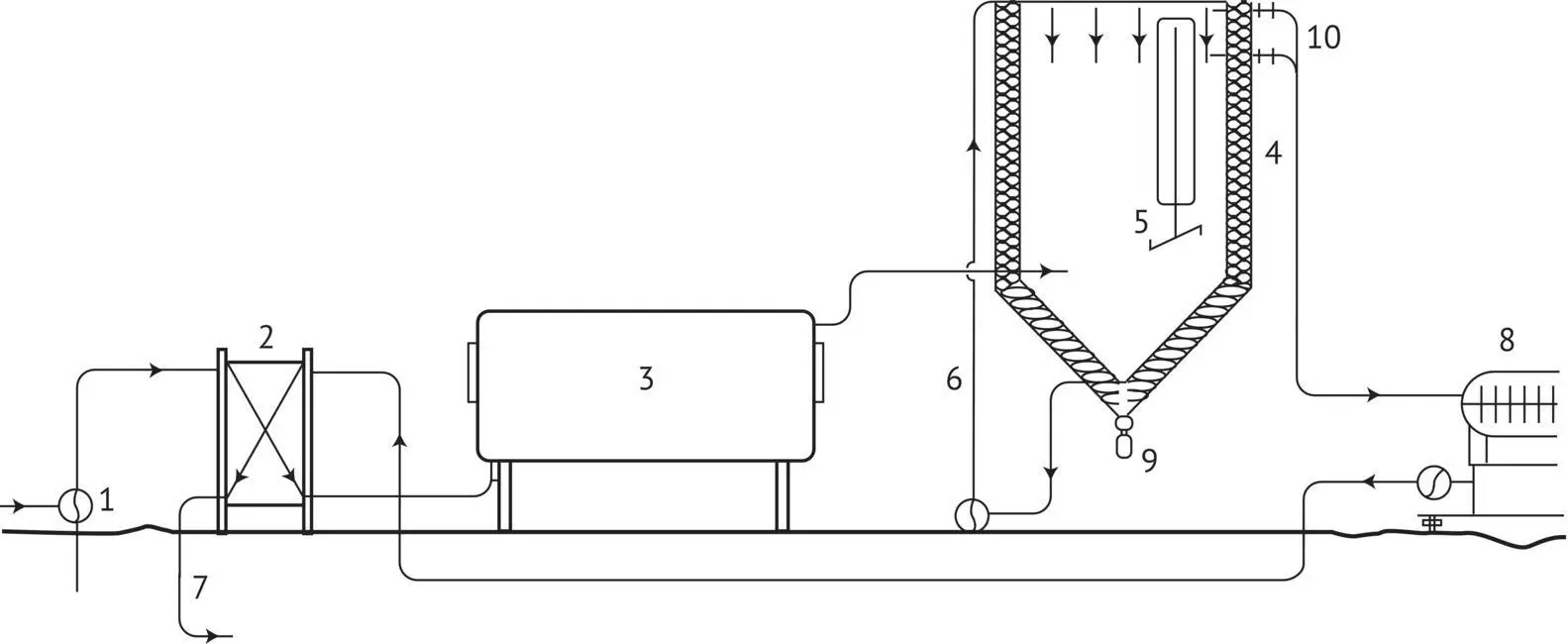
FIGURE 1.17 Schematic diagram of a continuous cold stabilization system: 1, intake of wine to be treated; 2, heat exchanger; 3, refrigeration system (with compressor, condenser, etc.); 4, insulation; 5, mechanical agitator; 6, recycling circuit (optional); 7, outlet of treated wine; 8, filter (earth); 9, drain; 10, overflow.
Agitation is partly provided by a tangential input of wine into the crystallizer. This creates turbulence in the bulk of the liquid and maintains at least the smallest crystals in suspension. The wine may also be mechanically agitated.
The throughput, i.e. the average time in the crystallizer, is defined according to the wine's initial state of supersaturation, as well as the type of preparatory treatment (fining, bentonite, etc.) received prior to artificial cold stabilization. The importance of preparation has already been mentioned ( Section 1.6.4).
The effectiveness of the three processes described above is generally satisfactory, although results depend on the type of wine (white or red), its alcohol content, and any previous treatment or fining.
It is true that, in contact treatments involving large‐scale seeding, the wine's background is less important. Indeed, enologists do not always have this information if the wine has been purchased from another winery. In any event, wine must be well prepared and, above all, properly clarified to ensure the effectiveness of rapid artificial cold stabilization treatments.
1.7.5 Preventing Calcium Tartrate Problems
Calcium tartrate is a relatively insoluble salt. It is 10 times less soluble than potassium bitartrate (see Section 1.5.1, Table 1.11). Independently of any accidental contamination, calcium added in the form of calcium bentonite for treating must or wine, calcium carbonate for deacidification purposes, or even as a contaminant in sucrose used for chaptalization, may cause an increase in the calcium tartrate content of wine. Combined with an increase in pH, this may put the wine into a state of supersaturation for this salt, leading to crystal deposits. Robillard et al . (1994) reported that crystallization of CaT was even observed in Champagne base wines with a particularly low pH. Ribéreau‐Gayon et al . (1977) considered there to be a real risk of tartrate deposits in the bottle when the calcium content is over 60 mg/l in red wine and 80 mg/l in white wine.
Stabilizing wines to prevent precipitation of calcium tartrate is not easy, as the crystallization of potassium bitartrate does not induce that of calcium tartrate, despite the fact that these two salts should logically crystallize together as they have the same crystal systems. In contrast, crystallization of CaT may induce that of KHT. The prevention of calcium tartrate precipitation is further complicated by the fact that the solubility of CaT (Postel, 1983) is not very temperature sensitive. Thus, CaT is just three times more soluble at 20°C than at −4°C.
Furthermore, according to Abgueguen and Boulton (1993), although the crystallization kinetics of CaT should be faster than those of KHT, the time required for spontaneous nucleation of CaT is much longer. It is therefore easier to understand why calcium tartrate precipitation generally occurs in wine after several years of aging.
On the basis of research into potassium bitartrate ( Figure 1.11), Vallée (1995) used measurements of electrical conductivity to define the width of the domain of supersaturation expressed in degrees Celsius, as well as the calcium tartrate saturation temperature of various types of wines. The low solubility of calcium tartrate indicates that saturation temperatures are likely to be much higher than those of potassium bitartrate.
To avoid the risk of calcium tartrate precipitation, the saturation temperature of white and rosé wines and vins doux naturels must be lower than 26°C to ensure that calcium tartrate deposits will not be formed if the wine is kept at 2°C for one month. The calcium tartrate saturation temperature for red wines must be below 35°C.
According to Postel (1983), the addition of 100 mg/l of metatartaric acid is capable of stabilizing a wine stored at 4°C for several months, so it does not suffer from crystalline deposits of CaT. Furthermore, the use of racemic acid (D‐L‐tartaric acid) or calcium L‐tartrate has been suggested for eliminating excess calcium (Ribéreau‐Gayon et al ., 1977). In both cases, the precipitation of calcium racemate, a highly insoluble salt, totally eliminates the cation. The treatment's effectiveness depends on the colloid content of the wine, as colloids hinder precipitation of the salt. These treatments are used to varying degrees in different wine regions depending on the types of wines produced.
Finally, ion exchange (Section 12.4.3) and electrodialysis (Section 12.5) are also processes for preventing calcium tartrate deposits.
1.7.6 The Use of Metatartaric Acid
In the processes described above, tartrate precipitations are prevented by eliminating the corresponding salts. It is also possible to envisage the addition of crystallization inhibitors.
Читать дальше













