Rhodococcus sp. are prominent for producing glycolipids like surface‐active molecules [48]. Peng et al. [49] reported the abundance of Rhodococcus erythropolis strain 3C‐9 in Xiamen Island coastal area soil, and they can remediate the oil‐contaminated soil in the area. A few scientists [13, 50] documented the production of various types of biosurfactants (glycolipid, polysaccharides, free fatty acids, and trehalose dicorynomycolate) by R. erythropolis [51] and Rhodococcus sp. [52].
Many scholars [53–57] reported the ability of different fungi to produce surfactants by using diverse nutrient sources. Earlier, it was documented that Candida sp. were the most common fungal species used for biosurfactant production compared to other fungi. Dubey et al. [58] outlined the application of Candida bombicola in sophorolipid production by using various carbon sources from industrial byproducts. Yarrowia lipolytica is a well‐known fungus used for the synthesis of bioemulsifiers based on lipid, carbohydrate, and proteins [59]. This polysaccharides bioemulsifier improved the cellular hydrophobicity throughout the developmental stages.
Yeast‐based biosurfactant research ( Candida sp. Pseudozyma sp. and Yarrowia sp.) has gained the growing interest of researchers [60]. The significant advantage of using yeast for biosurfactant production is its GRAS (generally recognized as safe) status, which includes Y. lipolytica , Saccharomyces cerevisiae , and Kluyveromyces lactis [61]. GRAS designated organisms are not toxic or pathogenic so their products can be used in the FMCG and pharma companies. Zinjarde et al. [62] demonstrated that extracellular bioemulsifier production occurs once cells reach a stagnant growth phase. It has been shown that Y. lipolytica NCIM 3589 screened from the ocean sites produces a cell wall‐associated emulsifier (a mixture of carbohydrate, lipid, and protein) using alkanes or crude oil. Fontes et al. [63] described another bioemulsifier type Yansan, produced from the Y. lipolytica wild strain, IMUFRJ 50682, in glucose‐enriched fermentation media of molasses.
Pseudozyma and Torulopis are the second most explored yeasts preceded by Candida for biosurfactant production. Stüwer et al. [64] reported the production of sophorolipids derived from Torulopis apicola on agricultural waste under lab conditions. Similarly, Vacheron et al. [65] also reported sophorolipid production from Torulopis petrophilum under different growth parameters by using waste materials. Morita et al. (2007) reported Candida antarctica and Pseudozyma rugulosa as Glycolipid (Mannosylerythritol lipids) producing yeasts, which have superior vesicle‐producing and surface‐active properties. Sarubbo et al. [66] reported biosurfactant production by using Y. lipolytica strain in the fermentation medium enriched with protein (47%), carbohydrate (45%), and lipids (5%), where glucose was the primary carbon source utilized for biosurfactant production.
3.5 Bacterial Growth Conditions
Biosurfactant production in a bioreactor is a complicated process involving the close monitoring of the conditions involved in it. First of all, it is crucial to choose a suitable culture method (i.e. Continuous, Batch, or Fed‐batch), which depends on the type of organism, product, and type of bioreactor [67–69]. Atlić et al. [70] reported that every culture method has specific biomass kinetics, substrates, or final product or byproduct concentrations ( Figure 3.2).
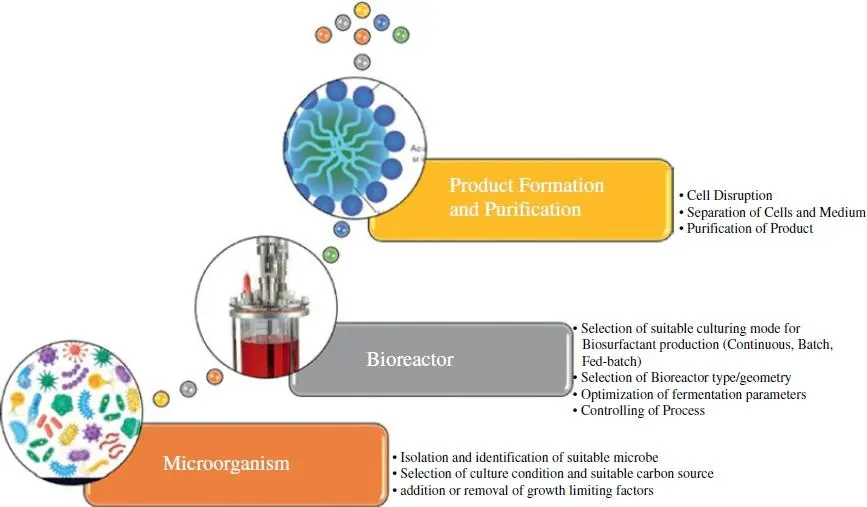
Figure 3.2 Key stages used for bioproduct formation in bioreactor.
For example, researchers [71] described an advanced method for surfactin production in the stirred‐tank bioreactor with the help of Aureobasidium pullulans LB 83. Their group used a centrally ordered facial model intending to evaluate the impact of aeration levels (0.1–1.1 per min) and sucrose concentrations (20–80 g/l) on total biosurfactant productivity. Their results reported an increase in tensoactivity of 8.05 cm in an oil spreading test and productiveness of 0.0838 cm/h when all parameters were used at high levels.
3.5.1 Continuous Cultures
The continuous cultivation of microbes is one of the methodology of growing significance [72]. This methodology is primarily characterized by constant microbial growth in continuous culture, i.e. a constant rate of growth in a constant environment. In continuous culture, parameters such as pH, substrate concentrations, metabolic products, and oxygen that eventually shift during the “batch cultivation” growth cycle are all constant; however, the experimenter may individually monitor and control them [73–75]. These attributes of the continuous culture method make it a desirable option for research while offering the industrial microbiologist several benefits in terms of more affordable production techniques ( Figure 3.3).

Figure 3.3 Diagrammatic presentation of one stage and two stage fermentation process for biosurfactant production.
The continuous culture of microbes is performed in bioreactors called chemostats [76]. Chemostat is a type of bioreactor to which freshly prepared substrate is constantly supplied, while culture liquid comprising remaining nutrients, microbial end products, and microbe culture is continually withdrawn simultaneously at the same rate to maintain a constant culture volume. By adjusting the rate at which freshly prepared culture medium is supplied to the bioreactor, the specific growth rate of microorganisms can be effectively managed under limits [77, 78].
There may be several types of the continuous bioreactor, for example:
1 Auxostat: An auxostat is a method that uses inputs from a microbial growth chamber analysis to monitor the constant media flowrate, keeping the calculation at a constant level [79].
2 Retentostat: Retentostat is a modified form of chemostat in which a filtration assembly is linked to the effluent line and thus recycles the biomass to the bioreactor [19].
3 Turbidostat: A turbidostat is a continuous cultivation system with input on the optical density and dilution rate of the culture vessel [80].
Batch fermentation is widely used in fermentation industries for the production of various microbial products, including vitamins, hormones, drugs, and secondary metabolites [81]. In the batch process, microorganisms and substrates are supplied into a bioreactor on a batch‐wise basis for product synthesis [82]. The batch approach is a simple way of conducting the fermentation and ensuring controlled environments inside a bioreactor. However, during the fermentation process, competitive changes may occur in microbial biomass, acid concentration, and byproduct concentration. The batch bioreactor consists of a mechanically agitated container with many other fittings, including gas sprayer, insulation jacket for regulating temperature shifts, pH meter, and air spargers [83, 84]. Despite the easiness of the batch process, batch fermentation incurs huge expenses and consumes a large amount of time spent on preliminary and post‐run operations, including bioreactors emptying, filling, and cleaning. In certain situations, such operations can take a much longer time than growing the microbial biomass [85].
Читать дальше














