7 Chapter 8Figure 8.1 Different types of photocatalytic overall water splitting systems including (a) one‐step photoexcitation, (b) first‐generation Z‐scheme, (c) second‐generation Z‐scheme, and (d) third‐generation Z‐scheme. HEP: hydrogen evolution photocatalyst; OEP: oxygen evolution photocatalyst; HEC: hydrogen evolution cocatalyst; OEC: oxygen evolution cocatalyst; CB: conduction band; VB: valence band; E g: energy band gap; NHE: normal hydrogen electrode; Ox: oxidant; Red: reductant.Figure 8.2 Representative material types for one‐step photocatalytic overall water splitting systems. (A) SrTiO 3with 18 facets and selective deposition of HER and OER cocatalysts. Source: Mu et al. [68]. Copyright 2016, the Royal Society of Chemistry.Figure 8.3 Representative Z‐scheme types for photocatalytic overall water splitting. (a) First‐generation Z‐scheme coupling ZrO 2/TaON(HEP) and BiVO 4(OEP) with Fe 3+/Fe 2+redox pairs as aqueous electron mediator and corresponding performance for 12 hours. Source: From Qi et al. [237]. © 2018 Elsevier.Figure 8.4 Different types of PEC for water splitting comprising (a) photoanode–cathode, (b) photocathode–anode, (c) photoanode–photocathode tandem cell, and (d) photovoltaic–photoanode (PV–PEC). Source: From Kim et al. [19]. © 2019 RCS.Figure 8.5 Photoanode–photocathode tandem cell for overall water splitting systems using (a) metal oxide‐based photoelectrodes (Cu 2O as HEP and Mo:BiVO 4as OEP) and produced photocurrent density with stoichiometric H 2and O 2evolution. Source: Reproduced with permission. Pan et al. [368]. Copyright 2018, Macmillan Publishers Limited, part of Springer Nature.Figure 8.6 Photoanode–photocathode tandem cell for overall water splitting systems using (A) PEC system composed of IrO x/n‐GaAs (photoanode) and Pt/Ti/Pt/Au/p‐GaAS (photocathode) and representative J – E curve. Source: Reproduced with permission. Kang et al. [395]. Copyright 2017, Macmillan Publishers Limited, part of Springer Nature.Figure 8.7 Representative PV–PEC devices for overall water splitting. (a) Inverted metamorphic multi‐junction (IMM) device structure with GaInP/GaInAs tandem PV cells coupled with RuO x–Pt and J–V measurements. Source: From Young et al. [403]. © 2017 Springer Nature.Figure 8.8 Representative PV–PEC devices for overall water splitting. (a) System composed of single‐junction perovskite solar cell and a nanocone/Mo‐doped BiVO 4/Fe(Ni)OOH photoanode, and respective J–V curve for the tandem device. Source: From Qiu et al. [325]. © 2016 Yongcai Qiu.Figure 8.9 Representative artificial (wireless) PV–PEC devices for overall water splitting. (a) Tandem CH 3NH 3PbI 3perovskite single‐junction solar cell with Co‐Ci incorporated H‐doped Mo/BiVO 4photoanode and corresponding gas evolution with calculated STH efficiency. Source: From Kim et al. [421]. © 2015 American Chemical Society.
8 Chapter 9Figure 9.1 Schematic illustration of the natural photosynthesis (a) and the artificial photosynthesis (b) using semiconductor as the photocatalyst.Figure 9.2 Band positions of some semiconductor photocatalysts and the redox potentials of CO 2reduction at pH 7 in aqueous solution. Source: Li et al. [12]. © 2014, Springer Nature.Figure 9.3 Schematic diagram of the slurry reactor (a) and the fixed bed reactor (b). Source: (a) Ola and Maroto‐Valer [17]. Licensed under CC BY 4.0. (b) Bakherad et al. [19]. © 2020, Royal Society of ChemistryFigure 9.4 (a) Schematic illustration of optical‐fiber photo reactor. (b) Light propagation in a TiO 2‐coated fiber reactor. Source: Wu et al. [20]. © 2008, Springer Nature.Figure 9.5 Dye‐sensitized photocatalytic reaction.Figure 9.6 Band alignment of bulk CdSe, CdSe QDs with a diameter of 2.5 nm, and TiO 2, as well as relevant redox potentials of CO 2and H 2O. Source: Wang et al. [25]. © 2010, American Chemical Society.Figure 9.7 Schematic diagram of the reduction of CO 2and water vapor into hydrocarbon fuels through a nanotube–catalyst array. Source: Varghese et al. [28]. © 2009, American Chemical Society..Figure 9.8 Semiconductor heterojunctions in the form of type I (a), type II (b), and type III (c).Figure 9.9 Schematic diagram of the photoexcitation process of AgBr/TiO 2composite under visible‐light irradiation.Figure 9.10 Schematic illustration of TiO 2with {001} and {101} surface heterojunction. Source: Yu et al. [31]. © 2014, American Chemical Society.Figure 9.11 Schematic illustration of the spatial separation of redox sites on the TiO 2photocatalysts prepared without HF (a), by adding a moderate amount of HF (b), and by adding a high amount of HF (c).Figure 9.12 Schematic diagram of charge transfer in a Z‐scheme semiconductor–semiconductor composite.Figure 9.13 Schematic diagram of Z‐scheme BiVO 4/C/Cu 2O nanowire arrays. Source: Reproduced with permission. Kim et al. [32]. © 2018, American Chemical Society.Figure 9.14 Comparison of the energy band positions and the related redox reaction potentials of g‐C 3N 4and ZnO. Source: Yu et al. [33]. © 2015, Royal Society of Chemistry.Figure 9.15 Schematic diagram of the solid–liquid interface structure.Figure 9.16 Potential comparison about the reduction of CO 2, H 2CO 3, and CO 3 2−ions into methanol. Source: Pan and Chen [38]. © 2007, Elsevier.Figure 9.17 Possible adsorption mechanism of CO 2on TiO 2surface. Source: Wu and Huang [40]. © 2010, Springer Nature.Figure 9.18 Schematic illustration of the charge transfer and separation in RGO–CdS nanorod system under visible‐light irradiation. Source: Yu et al. [41]. © 2014, Royal Society of Chemistry.Figure 9.19 Two proposed mechanisms for the photoreduction of CO 2to methane: formaldehyde (a) and carbine (b) pathways. Source: Izumi [42]. © 2015, American Chemical Society.Figure 9.20 Photocatalytic reduction of CO 2(86.65 kPa) by TiO 2(a) and Pd(1%)‐TiO 2(b). Source: Xiong et al. [44]. © 2017, Elsevier.Figure 9.21 Schematic mechanism of photocatalytic CO 2reduction over CQDs/OCN‐x under visible‐light irradiation. Source: Li et al. [45]. © 2019, Springer Nature.
9 Chapter 10Figure 10.1 Schematic diagrams for PEC CO 2reduction in water using a semiconductor as (a) photocathode, (b) photoanode, and (c) both photoanode and photocathode. (d) Schematic diagram for the device combining a photovoltaic cell with an efficient electrochemical catalyst for CO 2reduction. Source: Zhang et al. [18]. © 2018, Springer Nature.Figure 10.2 (a) Scanning electron microscope (SEM) image of HA‐Co 3O 4. (b) Upper layer microflower morphology. (c) Lower layer rhombus nanorod morphology. (d) Transmission electron microscopy (TEM) of petals on the flower; Inset: selected area electron diffraction (SAED) pattern of petal region. (e) High‐resolution transmission electron microscopy (HRTEM) of petal region. (f) Formate yields of HA‐Co 3O 4under PEC and EC under different negative potentials. Crystallographic modeling of Co 3O 4(g) {12 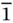 }, (h) {001}, (i) {110}, and (j) {111} facets with side view (left) and oblique view (right). Blue, light blue, and red spheres represent Co 3+, Co 2+, and O 2−, respectively. Source: Huang et al. [54]. © 2013, American Chemical Society.Figure 10.3 (a) Linear sweep voltammetry of the BCN 3.0electrodes loaded with or not loaded with cocatalysts. (b) Products analyses of photoelectrochemical reduction of CO 2over cocatalyst‐loaded BCN 3.0electrodes. Source: Sagara et al. [26]. © 2016, Elsevier.Figure 10.4 Periodic table depicting the primary reduction products in CO 2‐saturated aqueous electrolytes on metal and carbon electrodes. Source: White et al. [2]. © 2015, American Chemical Society.Figure 10.5 Schematic representations for (a) Co IIcomplex and (b) Fe IIcomplex for PEC reduction of CO 2. Source: Chen et al. [83]. © 2015, American Chemical Society.Figure 10.6 Schematic of (a) the ruthenium dye‐sensitized TiO 2nanoparticles with adsorbed CODH enzyme, which catalyzes the reduction of CO 2to CO. Source: Woolerton et al. [89]. © 2010, American Chemical SocietyFigure 10.7 Schematic image of (a) NiO–RuRe as photocathode and CoO x/TaON as photoanode. Source: Sahara et al. [30]. © 2016, American Chemical Society.
}, (h) {001}, (i) {110}, and (j) {111} facets with side view (left) and oblique view (right). Blue, light blue, and red spheres represent Co 3+, Co 2+, and O 2−, respectively. Source: Huang et al. [54]. © 2013, American Chemical Society.Figure 10.3 (a) Linear sweep voltammetry of the BCN 3.0electrodes loaded with or not loaded with cocatalysts. (b) Products analyses of photoelectrochemical reduction of CO 2over cocatalyst‐loaded BCN 3.0electrodes. Source: Sagara et al. [26]. © 2016, Elsevier.Figure 10.4 Periodic table depicting the primary reduction products in CO 2‐saturated aqueous electrolytes on metal and carbon electrodes. Source: White et al. [2]. © 2015, American Chemical Society.Figure 10.5 Schematic representations for (a) Co IIcomplex and (b) Fe IIcomplex for PEC reduction of CO 2. Source: Chen et al. [83]. © 2015, American Chemical Society.Figure 10.6 Schematic of (a) the ruthenium dye‐sensitized TiO 2nanoparticles with adsorbed CODH enzyme, which catalyzes the reduction of CO 2to CO. Source: Woolerton et al. [89]. © 2010, American Chemical SocietyFigure 10.7 Schematic image of (a) NiO–RuRe as photocathode and CoO x/TaON as photoanode. Source: Sahara et al. [30]. © 2016, American Chemical Society.
Читать дальше

 }, (h) {001}, (i) {110}, and (j) {111} facets with side view (left) and oblique view (right). Blue, light blue, and red spheres represent Co 3+, Co 2+, and O 2−, respectively. Source: Huang et al. [54]. © 2013, American Chemical Society.Figure 10.3 (a) Linear sweep voltammetry of the BCN 3.0electrodes loaded with or not loaded with cocatalysts. (b) Products analyses of photoelectrochemical reduction of CO 2over cocatalyst‐loaded BCN 3.0electrodes. Source: Sagara et al. [26]. © 2016, Elsevier.Figure 10.4 Periodic table depicting the primary reduction products in CO 2‐saturated aqueous electrolytes on metal and carbon electrodes. Source: White et al. [2]. © 2015, American Chemical Society.Figure 10.5 Schematic representations for (a) Co IIcomplex and (b) Fe IIcomplex for PEC reduction of CO 2. Source: Chen et al. [83]. © 2015, American Chemical Society.Figure 10.6 Schematic of (a) the ruthenium dye‐sensitized TiO 2nanoparticles with adsorbed CODH enzyme, which catalyzes the reduction of CO 2to CO. Source: Woolerton et al. [89]. © 2010, American Chemical SocietyFigure 10.7 Schematic image of (a) NiO–RuRe as photocathode and CoO x/TaON as photoanode. Source: Sahara et al. [30]. © 2016, American Chemical Society.
}, (h) {001}, (i) {110}, and (j) {111} facets with side view (left) and oblique view (right). Blue, light blue, and red spheres represent Co 3+, Co 2+, and O 2−, respectively. Source: Huang et al. [54]. © 2013, American Chemical Society.Figure 10.3 (a) Linear sweep voltammetry of the BCN 3.0electrodes loaded with or not loaded with cocatalysts. (b) Products analyses of photoelectrochemical reduction of CO 2over cocatalyst‐loaded BCN 3.0electrodes. Source: Sagara et al. [26]. © 2016, Elsevier.Figure 10.4 Periodic table depicting the primary reduction products in CO 2‐saturated aqueous electrolytes on metal and carbon electrodes. Source: White et al. [2]. © 2015, American Chemical Society.Figure 10.5 Schematic representations for (a) Co IIcomplex and (b) Fe IIcomplex for PEC reduction of CO 2. Source: Chen et al. [83]. © 2015, American Chemical Society.Figure 10.6 Schematic of (a) the ruthenium dye‐sensitized TiO 2nanoparticles with adsorbed CODH enzyme, which catalyzes the reduction of CO 2to CO. Source: Woolerton et al. [89]. © 2010, American Chemical SocietyFigure 10.7 Schematic image of (a) NiO–RuRe as photocathode and CoO x/TaON as photoanode. Source: Sahara et al. [30]. © 2016, American Chemical Society.
![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)









