(3.2) 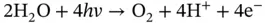
(3.3) 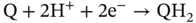
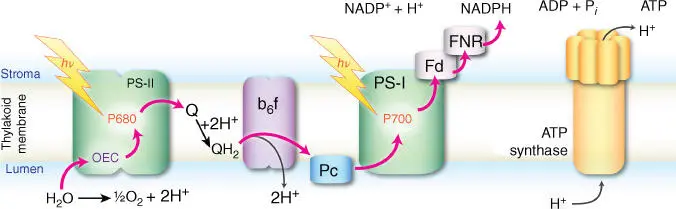
Figure 3.2Schematic membrane arrangement of enzymes involved in the light‐dependent reactions of oxygenic photosynthesis. Red arrows indicate the flow of electrons from the primary electron donor H 2O to the final electron acceptor NADP +aided by photoexcitation in photosystems II and I. Electron flow is tied to proton translocation across the membrane. The proton gradient created by accumulation of protons in the interior of the membrane powers the synthesis of ATP.
The reduced plastoquinone functions as a mobile electron carrier, transferring reducing equivalents from PS‐II to the cytochrome b 6 f complex. From there, another mobile carrier, the blue copper protein plastocyanin (PC), delivers electrons to photosystem I (PS‐I). A second light absorption/charge separation event at PS‐I, at a chlorophyll reaction center known as P700, promotes the electrons through chlorophylls (A 0), quinones (A 1), and a series of Fe–S centers (denoted F X, F A, and F B) to ferredoxin (Fd) and finally to ferredoxin–NADP reductase (FNR), which catalyzes the formation of NADPH. NADPH functions as the carrier of hydrogen to be used in CO 2fixation. The flow of electrons depicted in Figure 3.2is coupled to generation of a proton gradient across the membrane that drives the synthesis of energy‐rich ATP molecules by ATP synthase.
Photosynthesis can be viewed as consisting of four “working modules” [20]: the first module corresponds to a light‐harvesting setup that funnels excitation energy to the second module, the charge‐separating reaction center. Charge separation drives the transfer of electrons from an oxidative module (in oxygenic photosynthesis, the OEC) to a reducing module. In natural photosynthesis the Calvin–Benson cycle is used to reduce CO 2to carbohydrates [4]. This part of natural photosynthesis, i.e. the light‐independent CO 2fixation reactions, will be discussed in a later section of this chapter. From the perspective of bioinspired artificial photosynthesis, the light‐dependent reactions discussed above are a sufficient blueprint. Further mimicking the complete network of biological processes that culminates in synthesis of carbohydrates is not a strict requirement, although its study is certain to yield valuable insights and inspiration. The successful solar‐driven generation of reducing equivalents by artificial means would already represent a complete achievement: the “reducing module” endpoint of an artificial photosynthetic system could as well involve the production of H 2as solar fuel itself, or the utilization of reducing equivalents for other chemical transformations that are technologically important but do not directly mimic the CO 2fixation reactions of natural photosynthesis. For this reason, emphasis will be placed in the following on the finer details of the light‐dependent processes outlined above.
3.3 Light Harvesting and Excitation Energy Transfer
Light harvesting in natural photosynthesis is accomplished by pigment–protein complexes known as antenna complexes. These are associated with the photosystems, and their role is to collect as much as possible of the available light and funnel the excitation energy to them. The photosynthetic pigments are mostly bacteriochlorophylls, chlorophylls, and carotenoids (carotenes and xanthophylls). Figure 3.3shows some of the chlorophylls utilized by photosynthetic organisms. They differ in substituents on ring A at positions 2 and 3 and on ring B at position 7 of the chlorin system. Chlorin differs from the porphin tetrapyrrole in having a reduced D ring, while in bacteriochlorins the B ring is also reduced. The principal characteristic of the electronic structure of chlorophyll‐type pigments is the conjugated π orbital system that is delocalized over the aromatic rings. Differences in substituents such as those shown in Figure 3.3create slight variations in electronic structure that contribute to distinct optical properties by affecting the nature and energies of π–π* excitations, the lowest of which falls within the red or near‐infrared (NIR) region. Thus, among the four types of chlorophyll shown in Figure 3.3, with respect to the most common chlorophyll a (Chl a ) that has an absorption maximum ( λ max) at ca. 662 nm in vitro , Chl b is blueshifted ( λ max= 644 nm) and Chl d is redshifted ( λ max= 697 nm) [4]. Chl f was discovered only recently [21] and is the most redshifted chlorophyll known in oxygenic photosynthesis ( λ max= 707 nm). It has been suggested that its presence in key positions within PS‐I and PS‐II enables charge separation to occur with far‐red light [22].
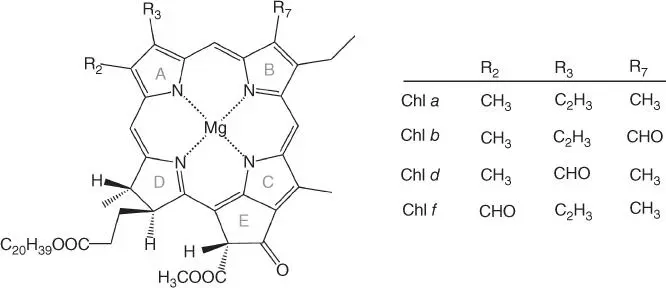
Figure 3.3Selected types of chlorophyll molecules encountered in natural photosynthesis.
The absorption profile of photosynthetic pigments is a crucial determinant of the ability of organisms to utilize the wavelengths of sunlight available within their particular ecological environment. Besides the availability of distinct molecular species, fine‐tuning the optical properties of individual (bacterio)chlorophylls can be achieved by (i) variable axial ligation at the Mg center, (ii) out‐of‐plane distortions of the (bacterio)chlorin ring, and (iii) the structured electrostatic environment of the protein matrix. These effects refer to the role of the protein in optimizing locally the properties of individual pigments. However, the truly amazing role of the protein in antenna complexes operates at the much larger scale of the precise and stable arrangement of arrays of pigment molecules (tens to hundreds of them) in terms of relative orientations and distances and in the control of their optical properties at the system level. The protein scaffold thus controls and optimizes the overall light‐harvesting profile of the antenna complex, maximizes the effective pigment concentration while avoiding concentration quenching, and ensures efficient and directional energy transfer to the charge separation site. At the same time, antenna complexes incorporate mechanisms of photoprotection, which become particularly important under conditions of high light intensity. Photoprotection refers principally to eliminating chlorophyll triplet states that can persist long enough to react with molecular oxygen and is typically achieved by incorporating carotenoids within the protein matrix. These can play a dual role as light harvesters at wavelengths not covered by chlorophylls, i.e. green and blue light, and as chlorophyll triplet‐state quenchers. There is a great diversity of antenna complexes in biology [23], characterized by variability in pigment composition and organization as well as in the mode of association with their respective reaction center complexes. Figure 3.4depicts selected examples of light‐harvesting complexes ranging from bacteria to higher plants.
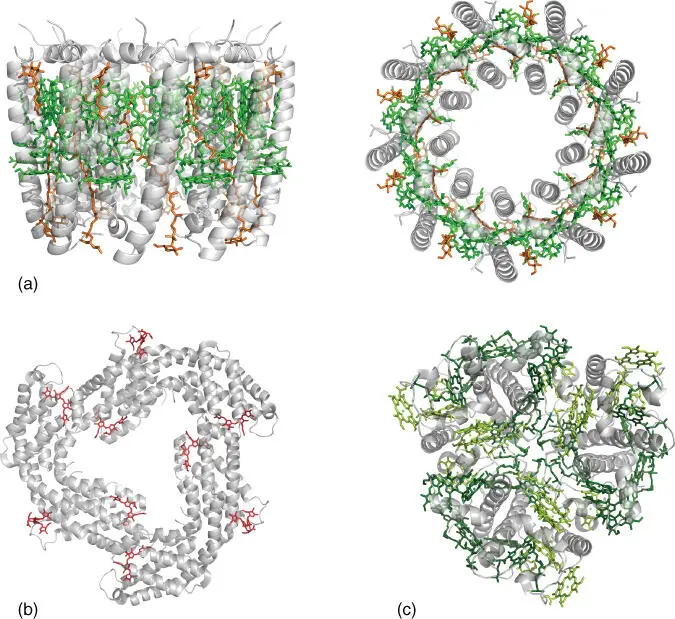
Figure 3.4(a) Side and top view of the light‐harvesting complex LH2 from the purple bacterium R. acidophila 10050 (PDB: 1KZU [24]), showing bacteriochlorophyll a pigments in green and carotenoids (rhodopin glucoside) in orange. (b) Rod structure of c ‐phycocyanin from the phycobilisome light‐harvesting antenna of cyanobacterium T. vulcanus (PDB: 3O18 [25]). (c) Light‐harvesting complex II (LHCII) from pea (PDB: 2BHW [26]), showing Chl a pigments in dark green and Chl b in light green.
Читать дальше

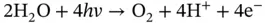
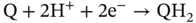




![Евгений Матерёв - Музеи… или вдохновляющая музыка The Chemical Brothers [litres самиздат]](/books/437288/evgenij-materev-muzei-ili-vdohnovlyayuchaya-muzyka-th-thumb.webp)









