The basic process that takes place in all reaction centers is described schematically in Fig. 1.4a. A chlorophyll‐like pigment (P) is promoted to an excited electronic state, either by direct photon absorption or, more commonly, by energy transfer from the antenna system. The excited state of the pigment is chemically an extremely strong reducing species. It rapidly loses an electron to a nearby electron acceptor molecule (A), generating an ion‐pair state P +A −. This is the “primary reaction” of photosynthesis. The energy has been transformed from electronic excitation to chemical redox energy. The system is now in a very vulnerable position with respect to losing the stored energy. If the electron is simply transferred back to P +from A −, a process called recombination, then the net result is that the energy is converted into heat and rapidly dissipated and is therefore unable to do any work. This pathway is possible because the highly oxidizing P +species is physically positioned directly next to the highly reducing A −species.
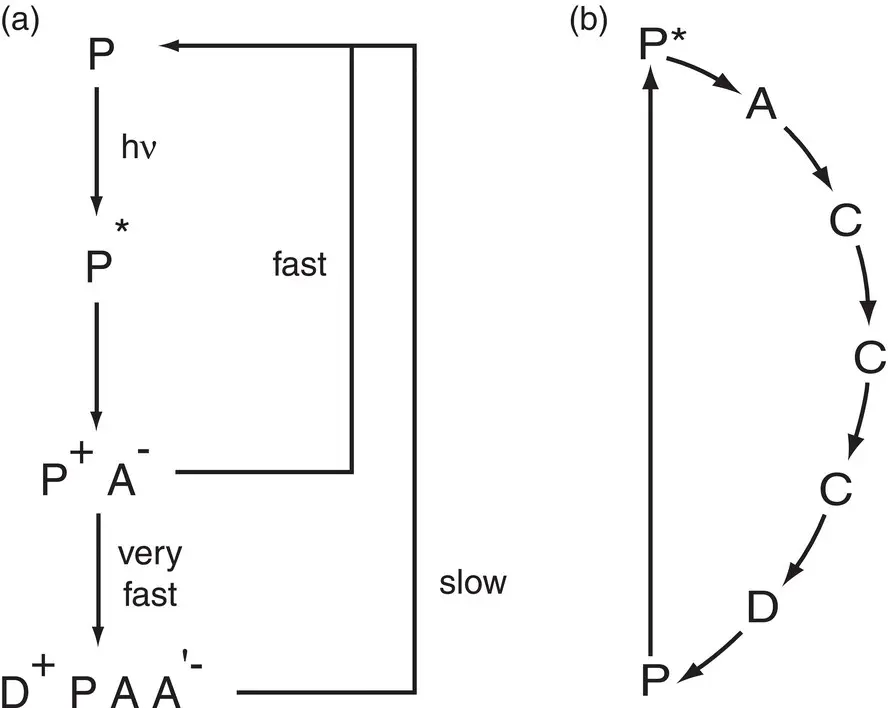
Figure 1.4 (a) General electron transfer scheme in photosynthetic reaction centers. Light excitation promotes a pigment (P) to an excited state (P*), where it loses an electron to an acceptor molecule (A) to form an ion‐pair state P +A −. Secondary reactions separate the charges, by transfer of an electron from an electron donor (D) and from the initial acceptor A to a secondary acceptor (A′). This spatial separation prevents the recombination reaction. (b) Schematic diagram of cyclic electron transfer pathway found in many anoxygenic photosynthetic bacteria. The terms fast, slow, and very fast are relative to each other. The vertical arrow signifies photon absorption: P represents the primary electron donor: D, A, and C represent secondary electron donors, acceptors, and carriers.
The system avoids the fate of recombination losses by having a series of extremely rapid secondary reactionsthat successfully compete with recombination. These reactions, which are most efficient on the acceptor side of the ion‐pair, spatially separate the positive and negative charges. This physical separation reduces the recombination rate by orders of magnitude.
The final result is that within a very short time (less than a nanosecond) the oxidized and reduced species are separated by nearly the thickness of the biological membrane (~30 Å; 1 Å = 0.1 nm). Slower processes can then take over and further stabilize the energy storage and convert it into more easily utilized forms. The system is so finely tuned that in optimum conditions the photochemical quantum yieldof products formed per photon absorbed is nearly 1.0 (see Appendix). Of course, some energy is sacrificed from each photon in order to accomplish this feat, but the result is no less impressive.
1.4.3 Stabilization by secondary reactions
The essence of photosynthetic energy storage is the transfer of an electron from an excited chlorophyll‐type pigment to an acceptor molecule in a pigment–protein complex called the reaction center. The initial, or primary, electron transfer event is followed by separation of the positive and negative charges by a very rapid series of secondary chemical reactions. This basic principle applies to all photosynthetic reaction centers, although the details of the process vary significantly from one system to the next.
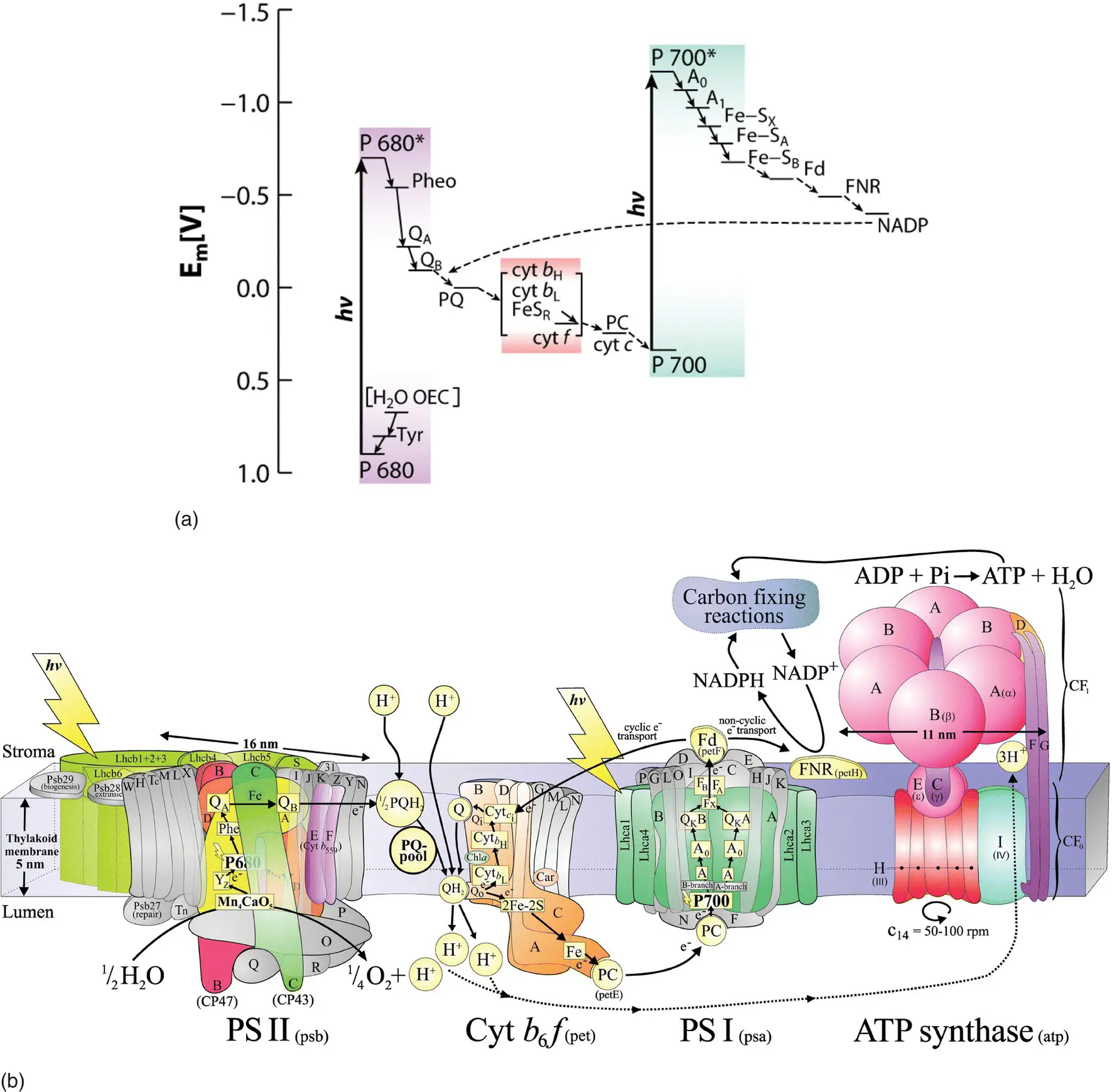
Figure 1.5 Schematic diagram of the noncyclic electron transfer pathway found in oxygenic photosynthetic organisms. The upper diagram (a) is an energetic picture of the electron transport pathway, incorporating the major reactions of photosynthesis into what is called the Z‐scheme of photosynthesis. The lower diagram (b) is a spatial picture, showing the major protein complexes whose energetics are shown in the Z‐scheme, and how they are arranged in the photosynthetic membrane. Neither view alone gives a complete picture, but together they summarize much information about photosynthetic energy storage.
Source: (a) Hohmann‐Marriott and Blankenship (2011) (p.532)/Annual Reviews. Reproduced with permission of Annual Reviews . (b) Courtesy of Dr. Jonathan Nield.
In some organisms, one light‐driven electron transfer and stabilization is sufficient to complete a cyclic electron transfer chain. This is shown schematically in Fig. 1.4b, in which the vertical arrow represents energy input to the system triggered by photon absorption, and the curved arrows represent spontaneous, or downhill, electron transfer processes that follow, eventually returning the electron to the primary electron donor. This cyclic electron transfer process is not in itself productive unless some of the energy of the photon can be stored. This takes place by the coupling of proton movement across the membrane with the electron transfer, so that the net result is a light‐driven difference of pH and electrical potential, or electrochemical potential gradient across the two sides of the membrane. This electrochemical potential gradient, called a protonmotive force, is used to drive the synthesis of ATP.
The more familiar oxygen‐evolving photosynthetic organisms have a different pattern of electron transfer. They have two photochemical reaction center complexes that work together in a noncyclic electron transfer chain, as shown in Fig. 1.5. The two reaction center complexes are known as Photosystems I and II. Electrons are removed from water by Photosystem II, oxidizing it to molecular oxygen, which is released as a waste product. The electrons extracted from water are transported via a quinone and the cytochrome b 6 f complex to Photosystem I and, after a second light‐driven electron transfer step, eventually reduce an intermediate electron acceptor, NADP +to form NADPH.
Protons are also transported across the membrane and into the thylakoid lumen during the process of the noncyclic electron transfer, creating a protonmotive force. The energy in this protonmotive force is used to make ATP (see Chapter 8).
Reaction centers and electron transfer processes in anoxygenic bacteria are discussed in more detail in Chapter 6, while these processes in oxygenic photosynthetic organisms are discussed in more detail in Chapter 7.
1.4.4 Synthesis and export of stable products
The final phase of photosynthetic energy storage involves the production of stable high‐energy molecules and their utilization to power a variety of cellular processes. This phase uses the intermediate reduced compound, NADPH, generated by Photosystem 1, along with the phosphate bond energy of ATP to reduce carbon dioxide to sugars. In eukaryotic photosynthetic organisms, phosphorylated sugars are then exported from the chloroplast. The carbon assimilation and reduction reactions are enzyme‐catalyzed processes that take place in the chloroplast stroma. These reactions are discussed in more detail in Chapter 9.
1 Béjà, O., Aravind, L., Koonin, E. V., Suzuki, M. T., Hadd, A., Nguyen, L. P., Jovanovich, S., Gates, C. M., Feldman, R. A., Spudich, J. L., Spudich, E. N., and DeLong, E. F. (2000) Bacterial rhodopsin: Evidence for a new type of phototrophy in the sea. Science 289: 1902–1906.
2 Ernst, O. P., Lodowski, D. T., Elstner, M., Hegemann, P., Brown, L. S., and Kandori, H. (2014) Microbial and animal rhodopsins: Structures, functions, and molecular mechanisms. Chemical Reviews 114: 126–163
Читать дальше














