Pathology of Genetically Engineered and Other Mutant Mice
Здесь есть возможность читать онлайн «Pathology of Genetically Engineered and Other Mutant Mice» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Pathology of Genetically Engineered and Other Mutant Mice
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:3 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 60
- 1
- 2
- 3
- 4
- 5
Pathology of Genetically Engineered and Other Mutant Mice: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Pathology of Genetically Engineered and Other Mutant Mice»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
An updated and comprehensive reference to pathology in every organ system in genetically modified mice Pathology of Genetically Engineered and Other Mutant Mice
Pathology of Genetically Engineered and Other Mutant Mice
Pathology of Genetically Engineered and Other Mutant Mice — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Pathology of Genetically Engineered and Other Mutant Mice», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
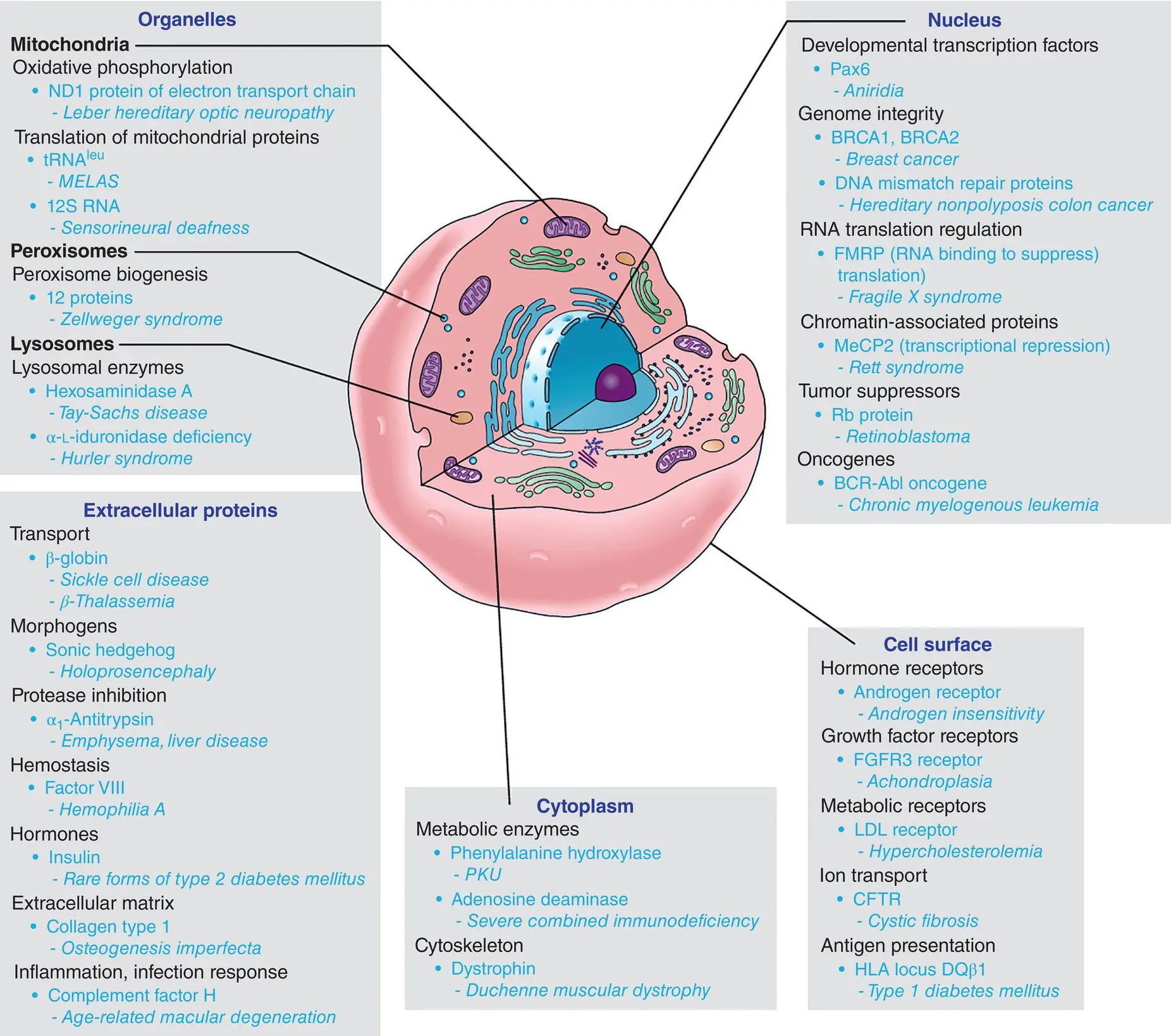
Figure 1.1 Classes of proteins associated with human genetic diseases.
Source: Nussbaum (2007). Reprinted with permission of Elsevier
Mouse Pathology – Nomenclature
The pathology of mice in research was first led by Thelma Dunn and Harold Stewart at the National Cancer Institute (NCI) at the NIH [11–13]. Both were MD pathologists who applied general rules of human pathology diagnosis to mice when possible. Although mouse pathology nomenclature does not follow any official designation, two organizations (INHAND; https://www.toxpath.org/inhand.aspand National Cancer Institute Mouse Models of Cancer Consortium Tumor Pathology Nomenclature) have provided international nomenclatures for specific tissues [2, 14]. Other published guides are included in each appropriate organ chapter [15, 16]. Many books and refereed publications on mouse pathology provide valuable information for pathologists and scientists [12,17–35] as well as web sites ( https://ntp.niehs.nih.gov/nnl; http://www.informatics.jax.org/frithbook; http://eulep.pdn.cam.ac.uk/~skinbase/index.php). Importantly, many of these references involve both DVMs and MDs, pathologists, and basic scientists, who integrate mouse and human disease nomenclature together to be state‐of‐the‐art. The pathology nomenclature used in this book generally reflects the NCI tumor pathology and INHAND general pathology nomenclatures. There are, however, no international or national standards for nomenclature that must be followed. These published nomenclatures are merely guidelines for use by scientists, pathologists, and journals. Each chapter author considered these guidelines and noted appropriate references for each organ and tissue.
Mouse Genetic Nomenclature
In contrast to pathology nomenclature, mouse genetic nomenclature is standardized. Chapter 3focuses on the details of the nomenclature system and discusses how it was developed. While the authors and editors have, for the most part, updated the nomenclature, not all authors were willing to do so. Regardless, one can and should use the Mouse Genome Informatics website to verify all genes and alleles, as discussed in the Chapter 3, to make sure they are working with the correct nomenclature and allelic mutations.
Tumor Pathology
It is known in human and mouse pathology that cancer pathogenesis follows a scheme of molecular pathogenesis and an associated histopathogenesis [14, 16, 36]. There have been numerous publications on the role of specific genes in tumor pathogenesis in humans and animals. It is not the intention of this book to review the role of all genes for which published information on mouse cancer models is available, but rather to provide samples of some of the more common and important genes that play important roles. GEM may involve a single gene and attempt to mimic the human genetic disorder, or GEM may represent non‐familial genetic changes in the pathways to disease including cancer. Tumor frequency data in wild‐type control mice, especially in aging studies, have often been reported in various strains and stocks [1, 7, 23, 35, 37, 38]. While these reports provide general background information on the frequency of cancer types in a wildtype inbred strain, the actual frequency will vary based on substrain, husbandry, and other factors, necessitating the use of adequate numbers of control mice for studies on frequency of cancers in GEMs.
Immunohistochemistry (IHC), Scoring, Image Analysis, and Other Supportive Research Pathology Techniques
A variety of special pathology techniques are important adjuncts to mouse research. These include immunohistochemistry (IHC), in situ hybridization (ISH), ultrastructure, imaging, image analysis, artificial intelligence, machine learning, and a variety of molecular techniques. Most chapters will include examples of these for the various tissues. Some publications offer reviews of the use of IHC in mice [39–42] and Internet sites offer IHC protocols ( http://tumor.informatics.jax.org/mtbwi/index.do, https://www.niehs.nih.gov/research/resources/protocols/protocols‐immuno/index.cfm) and whole slide images ( http://tumor.informatics.jax.org/mtbwi/lymphomaPathology.jsp).
Histopathology scoring (grading) of lesion type and severity can often be used for mouse models of disease, genetics, and preclinical development for drugs [6, 34,43–45]. Examples are given in some chapters. The newer fields of image analysis, artificial intelligence, and machine learning are growing quickly [46, 47] and allow scientific analysis of quantitative pathology data.
Publication of Erroneous Pathology Data: Inadvertent Fraud?
Publications involving mice and other animals sometimes include histopathology figures that do not show what is described in the figure legend and/or text [2, 48, 49]. This problem has occurred most often in publications that do not appear to include a pathologist as a co‐author, and in journals that are not pathology‐based. The absence of pathology support at a research institution may be due to cost, lack of pathology staff, and/or the desire of a scientist and his staff to attempt pathology on their own (“Do It yourself pathology”) [50]. These publications containing clearly erroneous findings may be due to lack of pathologists as reviewers of the submitted manuscript and/or a lack of the journal reviewers and editors who understand the value of accurate histopathology description and interpretation. Emails to authors and editors involved with the publication usually evoke no responses, and rarely concern even if there is a response. The only solution is for scientists and journals to understand the importance of pathology as a critically important medical specialty as part of doing research using mice.
Overall Organization of the Book
The Pathology of Genetically‐engineered Mice (GEM) and Other Mutant Mice is organized into introductory chapters on concepts on the use of mice in biomedical research and the critical need to include mouse pathology expertise. The mouse pathology chapters, by organ system or other topics, are generally organized into sections on anatomy and histology, special necropsy organ protocols, aging/spontaneous strain specific lesions, GEM, and references. The reference listings are not an extensive review of the topic since there are thousands of such references, but they do include recent reviews and relevant classic and current publications. Tables and figures call attention to important information on the mice and their applications.
Acknowledgments
The editors wish to thank the lead and co‐authors of all chapters for their valuable contributions and interactions with the editors.
This work was supported grants from the National Institutes of Health (R01‐CA089713 and P30‐CA034196 to JPS).
References
1 1 Brayton, C.F., Boyd, K.L., Everitt, J.L. et al. (2018). An introduction to pathology in biomedical research: a mission‐critical specialty for reproducibility and rigor in translational research. ILAR J. 59 (1): 1–3.
2 2 Cardiff, R.D., Ward, J.M., and Barthold, S.W. (2008). ‘One medicine—one pathology’: are veterinary and human pathology prepared? Lab. Invest. 88 (1): 18–26.
3 3 Holland, E.C. (ed.) (2004). Mouse Models of Human Cancer. Hoboken, NJ: Wiley‐Liss.
4 4 Proetzel, G. and Wiles, M.V. (eds.) (2016). Mouse Models for Drug Discovery: Methods and Protocols, 2e. New York, NY: Humana Press.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Pathology of Genetically Engineered and Other Mutant Mice»
Представляем Вашему вниманию похожие книги на «Pathology of Genetically Engineered and Other Mutant Mice» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Pathology of Genetically Engineered and Other Mutant Mice» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












