To describe the nature and scope of controls that are in place to ensure that human biomedical clinical trials are conducted in a manner which ensures the protection of human subjects participating in them and the scientific integrity of the trial plan. Understanding the nature, scope and reach of the regulatory controls as well as their enforceability is essential to applying the GCP principles.
The regulatory environment within which human biomedical clinical trials are conducted is a matrix composed of: (i) the policies, laws, and regulations which describe the requirements and guidance; (ii) the governmental jurisdictions (Federal, State, Country, Region e.g. EU) which are responsible for implementing the requirements through regulatory authorities; (iii) nongovernmental organizations, e.g. Academic Medical Centers (AMCs), which both sponsor clinical trials and set their own internal standards for how clinical trials will be conducted; and (iv) the professional organizations (e.g. WHO, WMA) which support, define, and interpret the requirements, standards, and guidelines for their professional members.
It is not always clear whether this matrix works together under a planned scenario but the end result is that if a commercial sponsor, medical establishment, or individual practitioner wishes to conduct experimental biomedical studies in humans, that activity receives considerable oversight and review. There are complexities which the regulatory environment matrix introduces to the conduct of clinical trials; therefore it is important to understand the makeup of the regulatory environment. For example conducting trials in conformance with ICH E6(R2) does not guarantee that a trial meets all of the applicable regulatory standards called for by the country or region where the study is performed but it is probable that the use of the those guidelines would ensure that 90% or more of applicable requirements will be met.
Let us begin the examination of the regulatory environment by reviewing each of the four part matrix as depicted in Figure 2.1.
2.4 Laws, Regulations, and Policies
The enforceable requirements governing human biomedical clinical trials are found in the written laws (statutes) and implementing regulations. These laws and regulations, which are based on governmental policies provide the societal backbone for the authorities to regulate and affect control over human biomedical clinical trial activities. The laws are written by the elected legislatures which in the United States is the Congress. Congress is composed of the Senate and House of Representatives. The laws are passed by the Legislature, i.e. Congress and approved by the President and as needed interpreted by the US Supreme Court. Laws however are very high level statements and call for implementing details. Once in place, USA laws are implemented by the executive departments who report to the President. In the United States, the Federal Food Drug and Cosmetic Act (FFDCA) is a primary governing statute and the implementing regulations are developed/written by staff in the Food and Drug Administration (FDA) which is part of the Department of Health and Human Services (DHHS). The regulations are memorialized in the US Code of Federal Regulations (CFR). Regulations are developed according to the Administrative Procedures Act of 1946 (APA) which calls for agencies such as the FDA/DHHS to (i) keep the public informed of their organization, procedures and rules; (ii) provide for public participation in the rulemaking process; (iii) to establish uniform standards for the conduct of formal rulemaking and adjudication; (iv) to define the scope of judicial review. The rules developed pursuant to the APA have the force and effect of law, i.e. they are enforceable. There are sanctions and penalties if the rules are not followed.
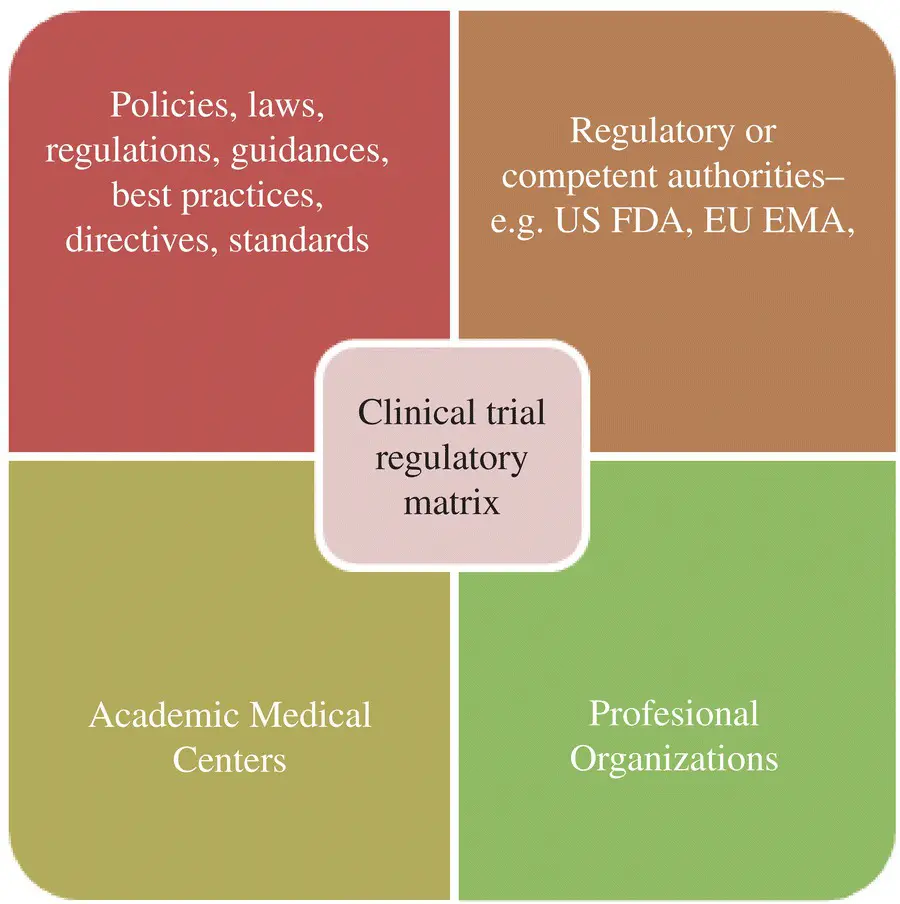
Figure 2.1 The clinical trial regulatory matrix.
The rules applicable to the conduct of clinical trials in the USA are found in Title 21 of the Code of Federal Regulations. The FDA considers the following Parts to be GCP:
21 CFR Part 50 – Protection of Human Subjects
21 CFR Part 54 – Financial Disclosure by Clinical Investigators
21 CFR Part 56 – Institutional Review Boards (IRBs)
21 CFR Part 312 – Investigational New Drug Application (prescription drugs)
21 CFR Part 812 – Investigational Device Exemptions (medical devices)
These regulations define the must do ground rules that apply to regulated biomedical research from informed consent to the content of an application for authorization to conduct the trial in humans. There are additional regulations which have impact on drug development and clinical trials activities such as electronic records, good laboratory practices, and marketing applications.
In addition to the FDA regulations the DHHS has an organizational group titled the Office of Human Research Protections (OHRP) which has responsibility for regulations governing research funded or performed by the federal government. Specifically: 45 CFR Part 46 – Federal Policy for the Protection of Human Research Subjects – “the Common Rule.”
The title of the rule speaks for itself in terms of content. It applies to all US Federal government biomedical and behavioral research conducted or sponsored by over 15 Federal department and agencies.
Federal regulations do not stand completely alone in terms of governmental oversight of clinical trial requirements and each of the 50 States has rules which affect one or more aspects of the conduct of clinical trials. For example the age of consent for trial subjects is reflected in State rather than Federal laws. The full nature and scope of the Federal and State requirements will not be covered in this text. The important point is that regulatory requirements may be found in more than one source and sponsors of clinical trials need to be cognizant of them all. ICH E6(R2) 5.1.1 speaks to that expectation.
Another well‐known example of the law and regulation framework is that of the European Union. The European Union’s approach is a bit different than the USA due to its regional construct but the legal arrangement resembles that of the USA. The European Union currently comprises 28 Member States. There is a central government organization, the European Council (EC) located in Brussels, Belgium. The EC, in conjunction with the European Parliament issues Directives which each of the member states are expected to implement or place into law and regulation within their own country’s legislative/governmental system. With regard to GCP the EC issued Directive 20/2001/EC – The Clinical Trial Directive in 2001. In 2005, the EC issued the Good Clinical Practice Directive – 2005/28/EC. These two directives were instrumental in closing several gaps which existed in the regulation of clinical trial activities in the EU member states. These Directives specifically called for ICH E6(R2) to be taken into account in terms of clinical trial regulation. Many people therefore view ICH E6(R2) as essentially a legal requirement in the EU. While changes are underway in several aspects of the regulatory framework established under the two EU Directives they have served as the pillars of regulation for human clinical trials involving pharmaceutical drugs in the EU. Notwithstanding expected changes in other aspects of clinical trial regulation ICH E6(R2) will continue to be the key criteria for conduct of a clinical trial.
It is useful to point out that the regulation of human clinical trials involving medical device products in the European Union is addressed under separate Directives e.g. 93/42/EEC. The overall regulation of medical devices in the European Union is also in the process of change.
Читать дальше













