Isotopic Constraints on Earth System Processes
Здесь есть возможность читать онлайн «Isotopic Constraints on Earth System Processes» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: unrecognised, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:Isotopic Constraints on Earth System Processes
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
Isotopic Constraints on Earth System Processes: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «Isotopic Constraints on Earth System Processes»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
Volume highlights include: Isotopic Constraints on Earth System Processes
The American Geophysical Union promotes discovery in Earth and space science for the benefit of humanity. Its publications disseminate scientific knowledge and provide resources for researchers, students, and professionals.
Isotopic Constraints on Earth System Processes — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «Isotopic Constraints on Earth System Processes», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
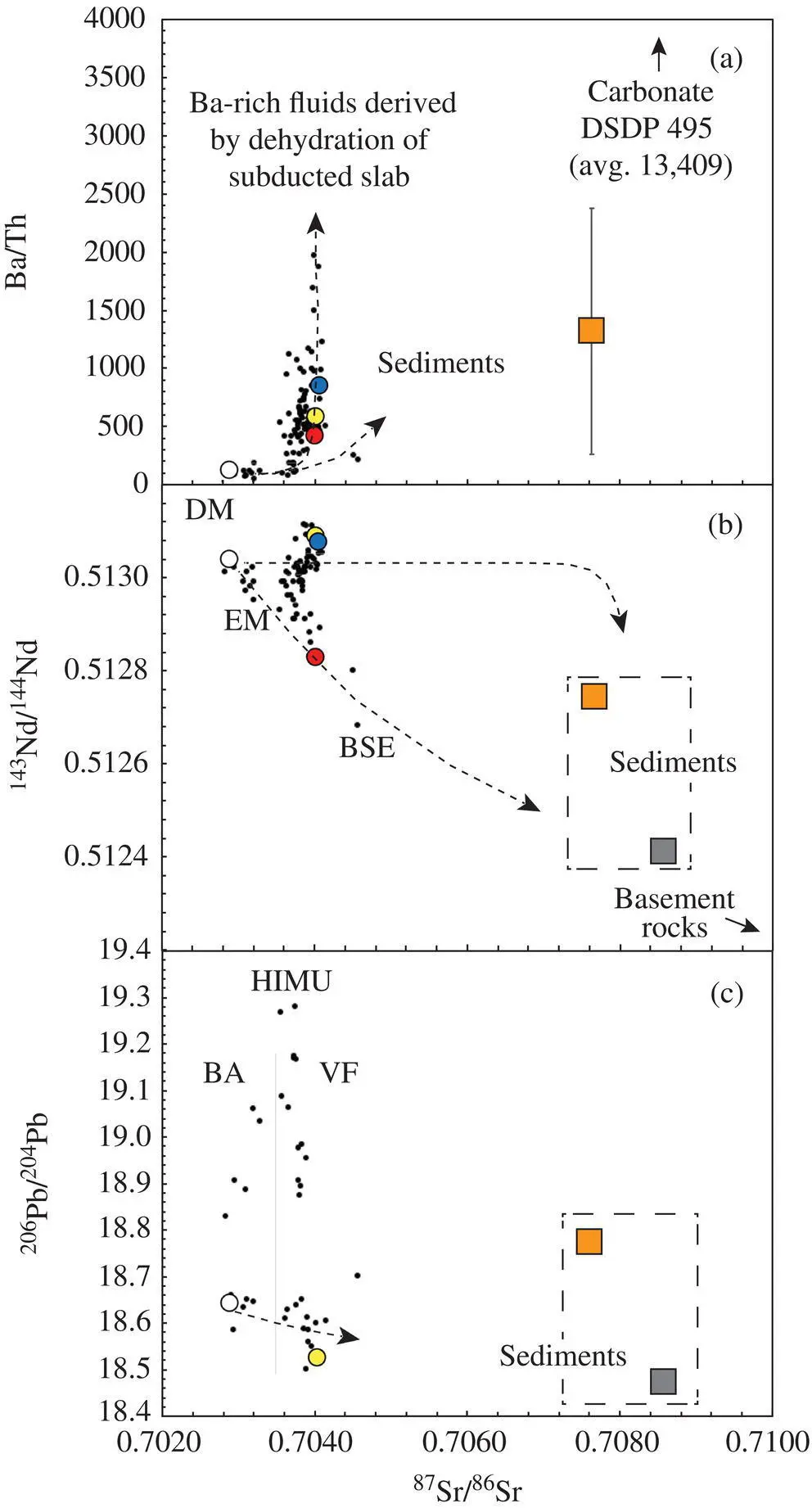
Figure 3.4 Ba/Th‐ 87Sr/ 86Sr (a), 143Nd/ 144Nd‐ 87Sr/ 86Sr (b), and 206Pb/ 204Pb‐ 87Sr/ 86Sr (c) diagrams for Central American volcanic arc lavas. All samples from the volcanic front (VF) have geochemical signatures interpreted to be elevated above values for back‐arc lavas (BA) by the addition of a sedimentary subducted component, e.g., enriched in 87Sr/ 86Sr. Back‐arc lavas including YO1 remain within the mantle field, reflecting mixtures of MORB‐like depleted mantle and HIMU, enriched mantle (data and illustrative mixing curves from Carr et al., 1990; Feigenson & Carr, 1986; Feigenson et al., 2004; Patino et al., 1997, 2000). Orange and gray squares represent hemipelagic and carbonate DSDP 495 sediment compositions, respectively.
3.4.2. Calcium Isotopic Record of Mantle‐Derived Rocks
Most mantle‐derived rocks and peridotites studies report calcium isotope compositions similar to BSE (e.g., Amsellem et al., 2019; Ionov et al., 2019; Kang et al., 2017; Simon & DePaolo, 2010). There are reports of mantle rocks including peridotites that have distinctly heavy δ 44Ca isotopic compositions, e.g., Amini et al. (2009), Huang et al. (2010), Kang et al. (2017), and Lu et al. (2019), and a few that are distinctly light, e.g., Amsellem et al. (2019) and Zhao et al. (2017). In order to investigate this variability, Huang et al. (2010) measured mineral separates from mantle rocks and found that δ 44Ca in orthopyroxenes (opx) are significantly heavier than their associated clinopyroxenes (cpx) by 0.36–0.75‰. The magnitude and sign of the measured differences are generally consistent with first principles equilibrium intermineral isotope fractionation calculations that fundamentally depend on calcium concentration (i.e., Ca—O bond strength) and temperature (Antonelli et al., 2019a; Wang et al., 2017). In detail, calcium isotope fractionation between opx and cpx (Δ 44/40Ca opx‐cpx) less than ~0.26‰ and greater than ~0.60‰ is likely related, at least in part, to disequilibrium calcium isotopes effects such as metasomatic metamorphism (Zhao et al., 2017). Disequilibrium effects have also been reported for volcanic settings during rapid crystal growth (Antonelli et al., 2019b) and between opx and cpx and with other minerals during granulite facies and ultrahigh‐temperature metamorphism (Antonelli et al., 2019a). In these cases, calcium concentration likely plays an important role, i.e., it is lower by ~1/32 in opx compared to cpx, and may be more easily affected by isotope fractionation governed by diffusive loss (or gain) of calcium. This would explain why cpx tends to have compositions closer to BSE but why opx compositions can vary wildly – opx as high as δ 44Ca ~6 has been found in mafic granulite samples (Antonelli et al 2019a). Interestingly, a recent investigation using calcium isotope signatures of carbonatite and silicate metasomatism and melt percolation found little evidence for calcium isotopic heterogeneity and concluded that metasomatism tends to decrease δ 44Ca values of metasomatized mantle materials, but that its effects are usually limited (≤0.3‰) (Ionov et al., 2019).
3.4.3. Calcium Isotopes Exhibit no Evidence for Carbonate Sediment Recycling at Subduction Zones
In the studied Central American arc magmas, I found no evidence for calcium isotopic heterogeneity and thus no evidence for carbonate recycling or any isotopic fractionation related to subduction. This is the case despite the fact that I selected rocks that have both little to no geochemical evidence for sediment subduction, i.e., YO1 has MORB‐like trace element signatures and depleted mantle (DM) radiogenic isotope compositions, and rocks with strong trace element and radiogenic isotope signatures for carbonate sediment subduction ( Fig. 3.2).
To date, resolvable radiogenic calcium isotopic signatures have not been observed in any oceanic or arc basalts (Huang et al., 2011; Marshall & DePaolo, 1989; Simon et al., 2009). This might not be surprising given the work of Caro et al. (2010) who, despite finding well‐defined excesses of 40Ca in some river waters draining into the ocean, report that no discernable effects of 40K decay, to within their reported analytical precision (~0.4 epsilon units, 2σ), exist in marine carbonate samples ranging in age from Archean to recent.
There have been recent studies of mantle‐derived rocks that find little evidence that recycling of carbonates affects the calcium isotope values of the mantle on a global or regional scale (Antonelli et al. 2019a; Ionov et al., 2019). However, other calcium isotope studies of primitive igneous rocks report evidence for recycling, e.g., Banergee and Chakrabarti (2019), Chen et al. (2018), Huang et al. (2011), Kang et al. (2016, 2017), and Liu et al. (2017). My results are significant since the trace element and radiogenic isotope signatures (e.g., high Ba/La, Ba/Th, 87Sr/ 86Sr, 206Pb/ 204Pb; see Figs. 3.2and 3.4) of Central American lavas suggest a significant contribution from subducted carbonates (Patino et al., 2000; Sadofsky et al., 2008). The geochemical decoupling reported herein contrasts with the signatures reported for the ocean island basalts studied by Huang et al. (2011). In the Huang et al. (2011) study, stable mass‐dependent calcium isotope signatures vary and correlate with other geochemical parameters (i.e., Sr/Nb and 87Sr/ 86Sr) used to support the interpretation that Hawaiian lavas represent recycling of ancient calcium bearing surface materials.
All samples from the volcanic front (VF) are interpreted to be elevated in their Pb and Sr radiogenic isotopes above values for back‐arc lavas (BA) by the addition of a sedimentary subducted component (see Fig. 3.4; Carr et al., 1990; Feigenson & Carr, 1986; Feigenson et al., 2004; Patino et al. 1997, 2000). Back‐arc lavas including YO1 remain within the mantle field, reflecting mixtures of MORB‐like depleted mantle (DM) and enriched mantle (HIMU). The potential sedimentary contribution to the arc magmas is believed to be uniform from Guatemala through northern Costa Rica and the sedimentary sequence has been well‐documented by the Deep Sea Drilling Program (DSDP); see Patino et al. (2000). The lower section of the sedimentary sequence consists of middle‐lower Miocene chalky carbonate ooze and manganiferous chalk and chert that are on average ~50 wt. % CaO (von Huene et al., 1982).
Subducted carbonate sediments along the Central American trough have compositions (Ba/La avg= 244, Sr/Nb avg= 3418, 87Sr/ 86Sr avg= 0.7086) that can produce a distinct signature in the arc basalts (Patino et al., 2000; Sadofsky et al., 2008). The calcium isotopic composition of this sediment has not been measured, but modern carbonate ooze (DSDP 590B) has a δ 44Ca = –0.36 ± 0.15‰ (2σ) (Fantle & DePaolo, 2005), similar to the modern riverine inputs to the oceans (DePaolo, 2004); see Fig. 3.3. The calcium isotope homogeneity among the studied arc magmas is particularly notable when one considers the fact that they exhibit a large range in their sediment contribution signatures. They exhibit compositions that range from non‐existent levels typical of MORB up to those near BSE in these sediment signatures (e.g., Ba/La varies from ~4 to 117, Fig. 3.2; Sr/Nb varies from 16–328; and 87Sr/ 86Sr varies from 0.7029–0.7041, Fig. 3.4) compared to the more limited range recorded by the Hawaiian tholeiites (Sr/Nb = 25–55, 87Sr/ 86Sr = 0.7035–0.7042), considered by Huang et al. (2011) to reflect ancient carbonate recycling. These observations imply that some traditional geochemical signatures for carbonate sediment subduction in arc magmas are at odds with their calcium isotopic signatures.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «Isotopic Constraints on Earth System Processes»
Представляем Вашему вниманию похожие книги на «Isotopic Constraints on Earth System Processes» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «Isotopic Constraints on Earth System Processes» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












