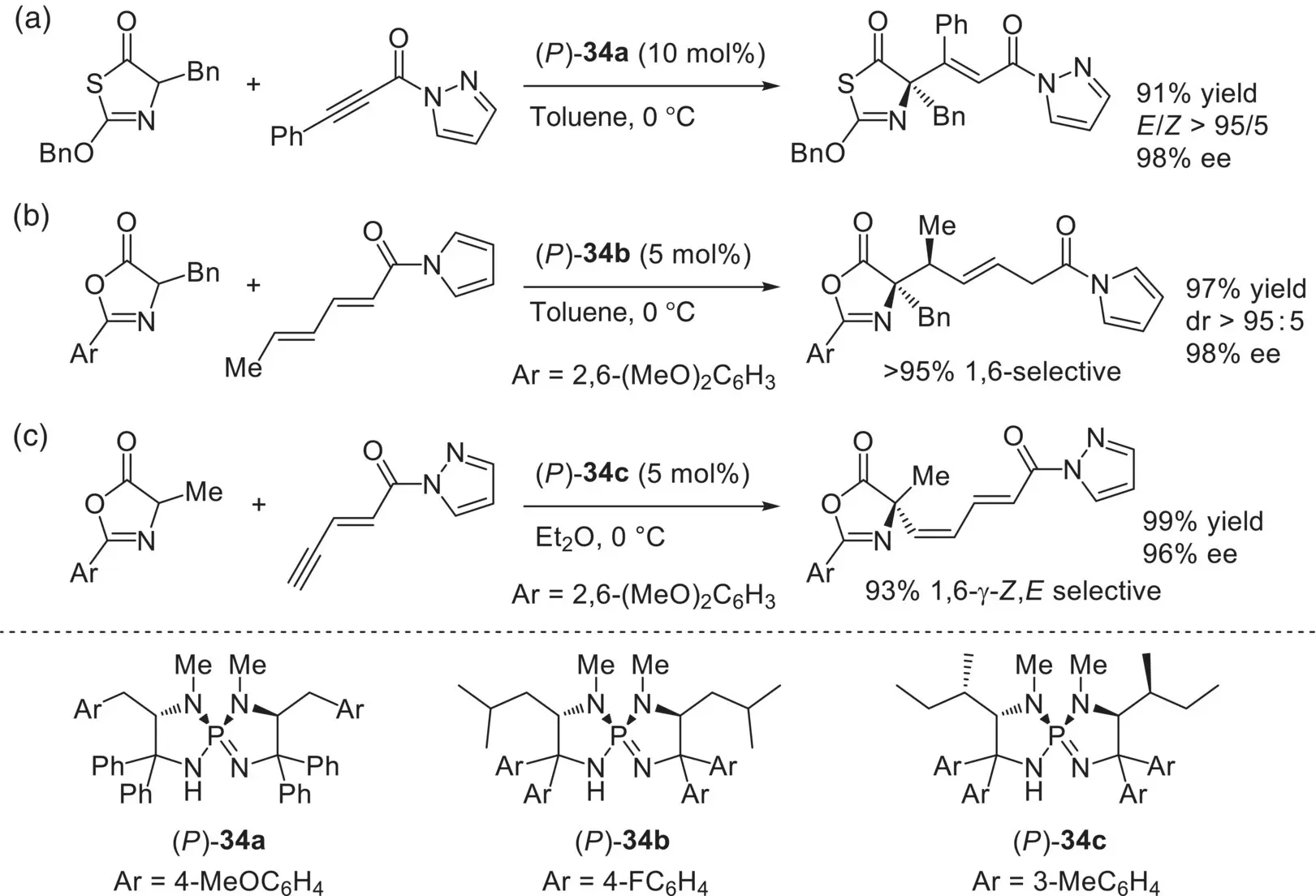
Scheme 3.50. Enantioselective Michael addition reactions catalyzed by ( P )‐ 34. (a)
Source: Based on [98].
(b)
Source: [99].
(c)
Source: Based on [100].
Furthermore, this class of chiral catalysts was successfully utilized in the development of new types of catalytic enantioselective reactions. For instance, Ooi, Johnson, and co‐workers developed the enantioselective aldol‐type reaction of α‐hydroxy phosphonoacetates ( Scheme 3.51) [101]. This reaction involves a catalytic generation of the reactive glycolate enolate 36from an α‐hydroxy phosphonoacetate 35through the [1,2]‐phospha‐Brook rearrangement and the subsequent enantioselective addition to an aldehyde. The group also reported the related enantioselective three‐component coupling reaction of isatin derivatives, aldehydes, and dialkyl phosphites [102].
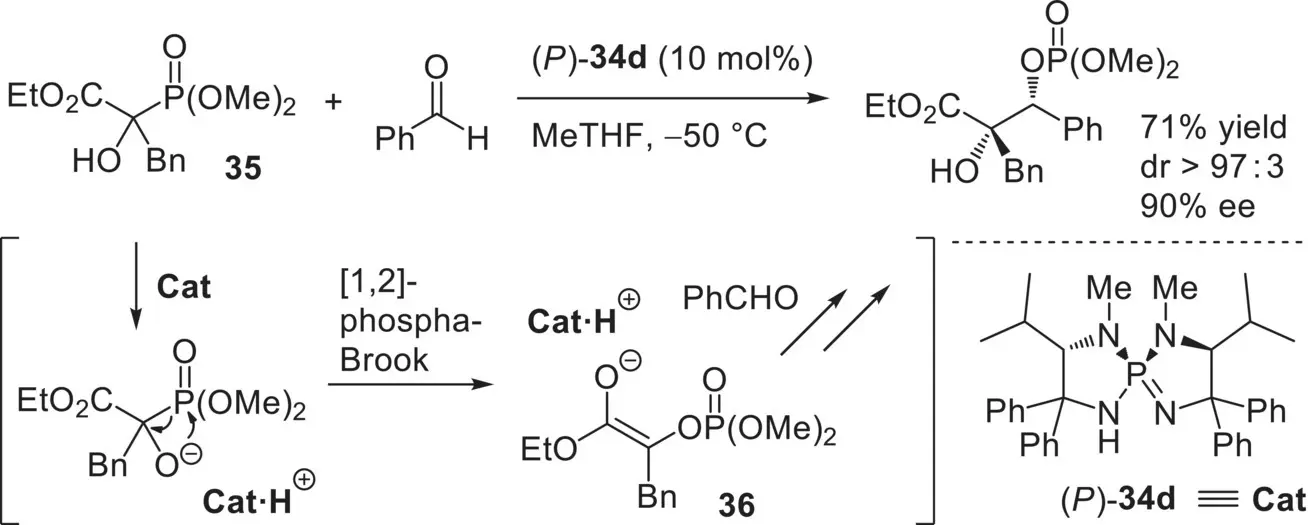
Scheme 3.51. Enantioselective aldol‐type reaction of α‐hydroxy phosphonoacetates catalyzed by ( P )‐ 34d.
Source: Based on [101].
As the other remarkable catalytic enantioselective reaction, Ooi and co‐workers developed the enantioselective Payne‐type oxidation of N ‐sulfonyl imines based on the combined use of H 2O 2and trichloroacetonitrile under the catalysis of ( P )‐ 34e( Scheme 3.52) [103]. In this reaction system, the reactive organic peroxy acid 37, which was catalytically generated in situ, was successfully controlled by the chiral catalyst molecule.
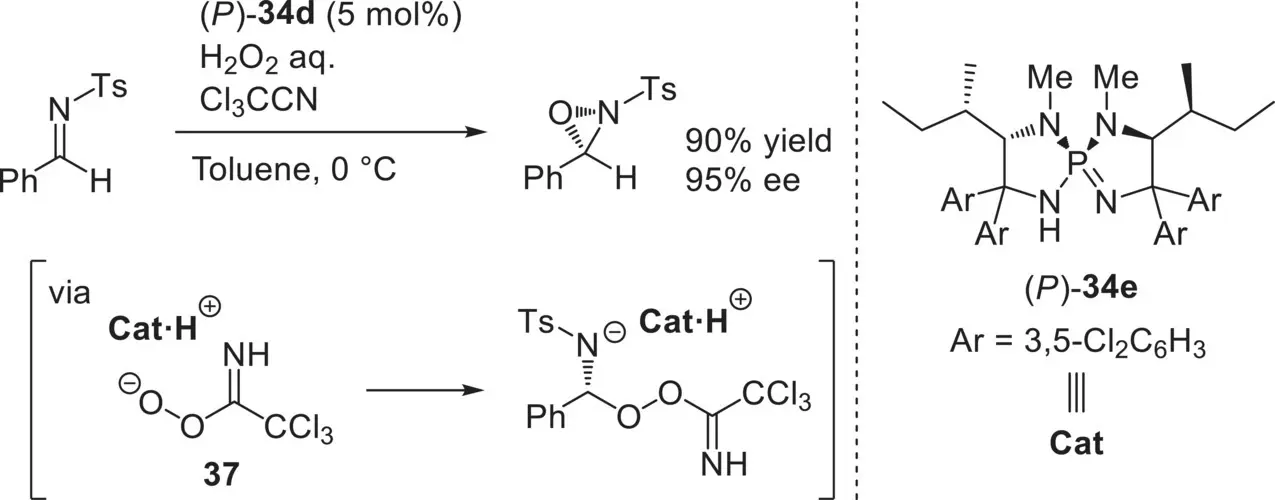
Scheme 3.52. Enantioselective Payne‐type oxidation of N ‐sulfonyl imines catalyzed by ( P )‐ 34e.
Source: [103].
3.4.4. Chiral Higher‐Order Phosphazene Catalysts
As described as well in the previous section, the expansion of the scope of pronucleophiles is one of the most important tasks in the field of asymmetric Brønsted base catalysis. The most direct approach for this purpose is the application of chiral organobases having strong basicity as a catalyst. The recent efforts for the development of chiral organobases with higher basicity than chiral tertiary amines, such as chiral guanidines, cyclopropenimines, triaryliminophosphoranes, and P1‐phosphazenes, have substantially advanced the field of asymmetric Brønsted base catalysis as described above. However, even with these chiral organosuperbase catalysts, the applicable pronucleophiles are still mainly limited to the compounds having high acidity because of their insufficient basicity. In order to overcome the intrinsic limitation of pronucleophiles, the development of chiral organobase catalysts possessing much higher basicity than aforementioned chiral organosuperbase catalysts, namely chiral “higher‐order” organosuperbase catalysts, is highly anticipated. In this context, Terada and co‐workers focused on higher‐order phosphazenes as a new platform for chiral organobase catalysts. Higher‐order phosphazenes, in which phosphazene or guanidine subunit(s) are introduced to the iminophosphorane core, possess much higher basicity than P1‐phosphazenes owing to the better delocalization of the positive charge formed through the protonation. Specifically, Terada and co‐workers designed and developed two types of chiral higher‐order phosphazene catalysts as a superb class of chiral organosuperbase catalysts that enable the expansion of the scope of pronucleophiles ( Figure 3.15).
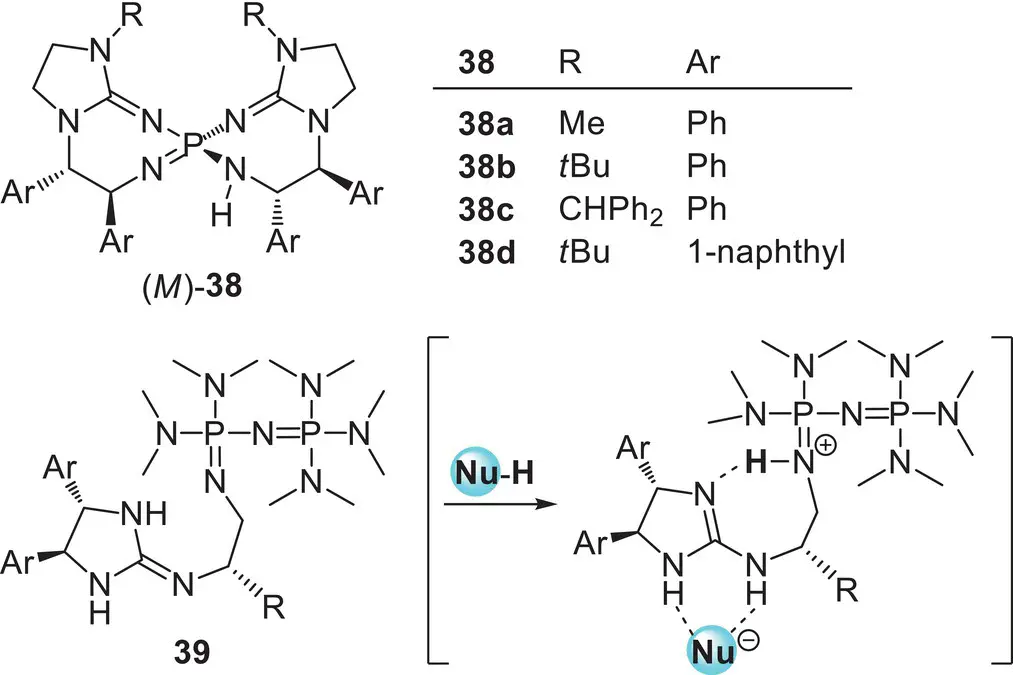
Figure 3.15. Chiral higher‐order phosphazene catalysts.
In 2013, Terada and Takeda developed chiral pseudo‐ C 2‐symmetric bis(guanidino)iminophosphorane catalysts ( M )‐ 38, in which two guanidine subunits are introduced to the central iminophosphorane core [104]. The characteristic feature of chiral bis(guanidino)iminophosphoranes is underscored by their helical chirality based on the 7,7‐membered spirocyclic system along with central chirality derived from chiral diamines. In addition, as similar to Ooi’s P1‐phosphazene catalysts, hydrogen bond donor and acceptor sites are arranged side by side around the central phosphorus atom. The catalytic performance of ( M )‐ 38as chiral “higher‐order” organosuperbases was validated in the enantioselective amination of 2‐alkyltetralone as a less acidic pronucleophile with azodicarboxylate ( Scheme 3.53).
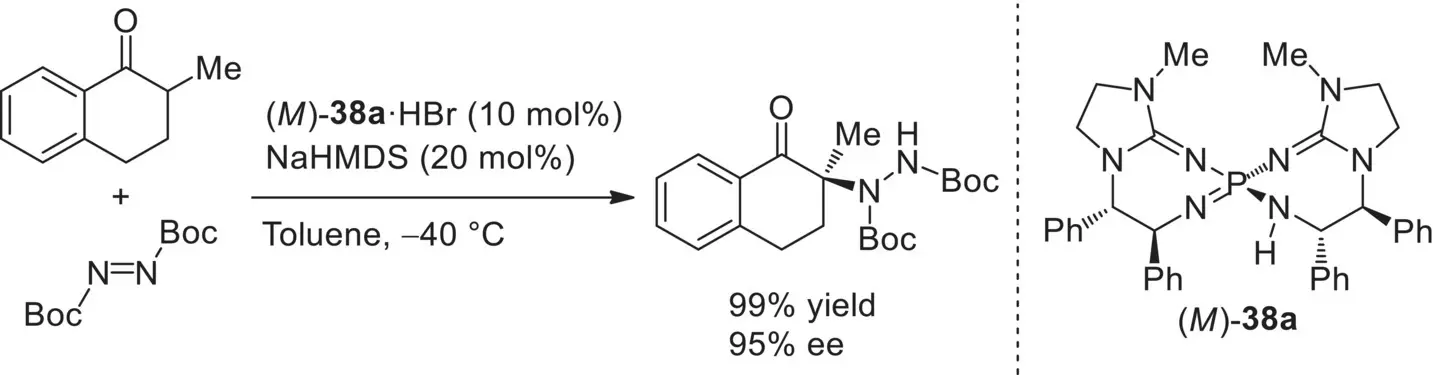
Scheme 3.53. Enantioselective amination of 2‐alkyltetralone with azodicarboxylate catalyzed by ( M )‐ 38a. Source: Based on [104].
By taking advantage of the strong basicity of ( M )‐ 38, Terada and co‐workers successfully developed a series of enantioselective addition reactions of less acidic pronucleophiles, such as 2‐alkoxycarbonyl 1,3‐dithianes, α‐alkylthionolactones, and 2‐benzylpyridine N ‐oxides ( Scheme 3.54) [105]. ( M )‐ 38was also applied to the enantioselective protonation through the hydrophosphinylation of 2‐vinyl quinoline N ‐oxides [106].
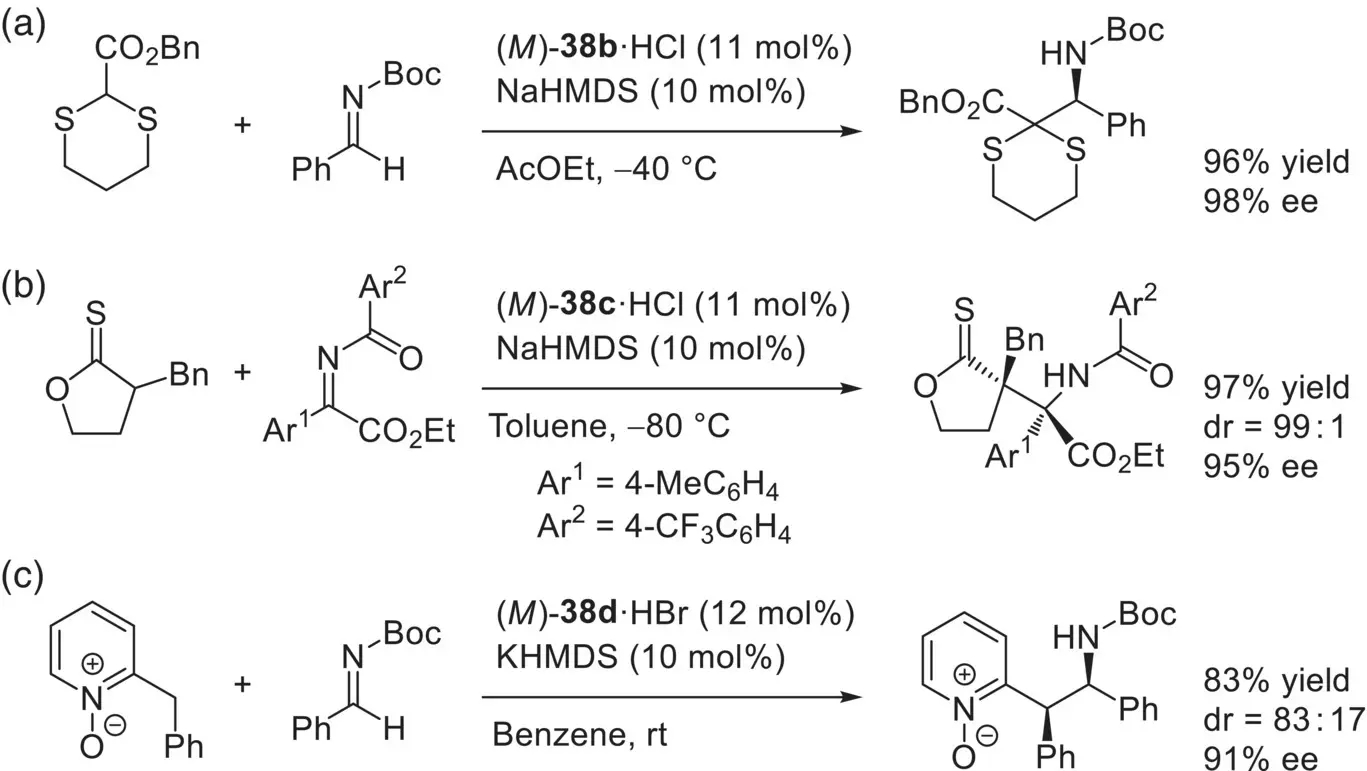
Scheme 3.54. Enantioselective addition reactions of less acidic pronucleophiles catalyzed by ( M )‐ 38.
Source: [105].
As the other remarkable application of ( M )‐ 38, Terada and co‐workers developed the enantioselective formal [3+2] cycloaddition of epoxysulfones with imines ( Scheme 3.55) [107]. This reaction involves the generation of alkoxide intermediate 40through the ring‐opening of epoxide and the subsequent enantioselective intermolecular addition to imine followed by the diastereoselective intramolecular aza‐Michael addition of chiral intermediate 41. In the tandem catalytic processes, the key roles of the chiral organosuperbase are: (i) facilitating the reaction with its high basicity, (ii) controlling the enantioselectivity in the addition of 40to imine, and (iii) assisting the diastereocontrol of the aza‐Michael addition of 41.
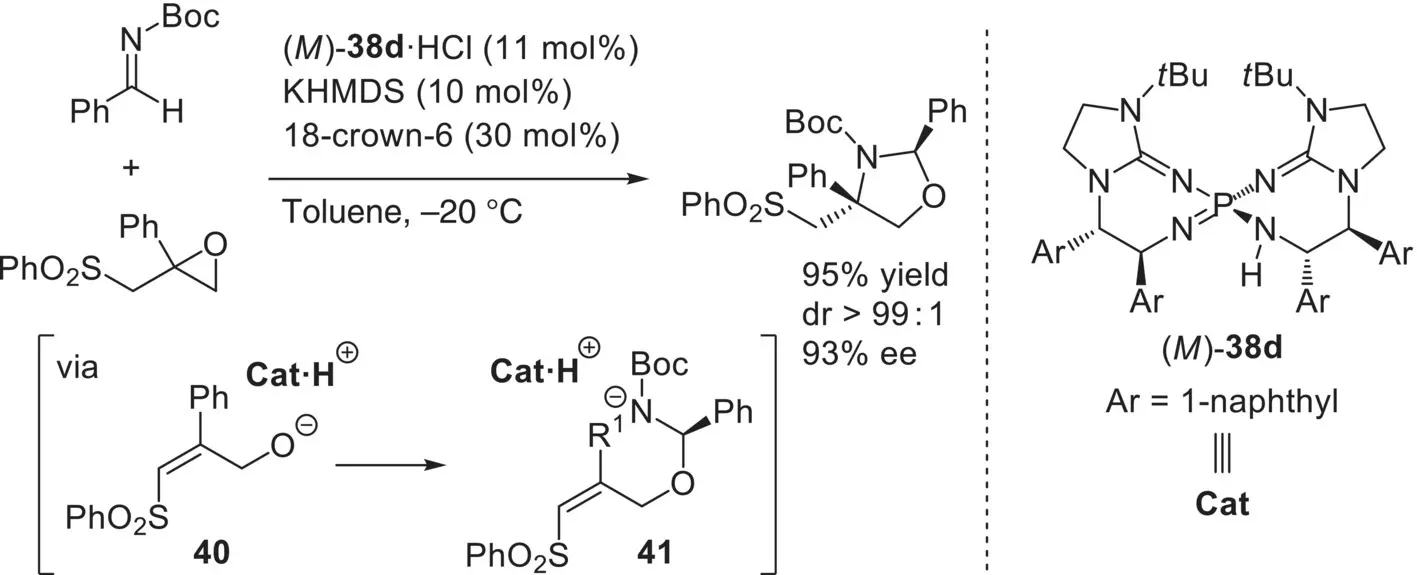
Scheme 3.55. Enantioselective formal [3+2] cycloaddition of epoxysulfones with imines catalyzed by ( M )‐ 38d.
Source: [107].
In 2020, Terada and co‐workers developed “chiral cooperative binary base catalysts” 39, which consist of two different organobase functionalities: a P2‐phosphazene as an organosuperbase and a chiral guanidine as a hydrogen bond donor unit for substrate recognition [108]. The molecular design is based on a conceptually new idea for a distinctive cooperative function by two organobases in a single catalyst molecule: the formation of a chiral cyclic structure with an intramolecular hydrogen bond between the two organobase functionalities in the conjugate acid form, which creates an effective chiral environment around the substrate recognition site by limiting the conformational flexibility ( Figure 3.15). The prominent catalytic activity of 39was demonstrated in the enantioselective direct Mannich‐type reaction of α‐phenylthioacetate as a less acidic pronucleophile ( Scheme 3.56a). The P3‐phosphazene‐based catalyst 42possessing enhanced basicity was also synthesized based on the molecular design. The catalyst could promote the reaction of much less acidic α‐phenylthioacetamide, which did not proceed with P2‐phosphazene‐based catalyst 39, albeit in moderate stereoselectivities ( Scheme 3.56b).
Читать дальше














