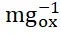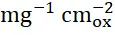Starfish-like phosphorus carbide nanotubes
Kistanov A. A. 1, Shcherbinin S. A. 2, Huttula M. 1, Cao W. 1
1 – Nano and Molecular Systems Research Unit, University of Oulu, Oulu, Finland
2 – Southern Federal University, Rostov-on-Don, Russia
stefanshcherbinin@gmail.com
Recently several allotropes of a novel two-dimensional material, phosphorus carbide (PC), have been predicted theoretically and some of them have already been successfully fabricated [1]. For one of these PC allotropes, α-PC, the possibility of its rolling to a PC nanotube (PCNT) at room temperature under compressive strain has been found [2]. These PCNTs of different sizes exhibit high thermal stability and possess well tunable band gap. In this work, PCNT obtained by the rippling of β 0-PC and β 1-PC monolayers along their armchair (APCNT) and zigzag (ZPCNT) directions are investigated in the framework of density functional theory.
It has been found that most of created β-PCNTs possess starfish-like structure (see Figure 1a). The dynamical stability of these β-PCNTs has been verified using ab initio molecular dynamics calculations conducted at 300 K. It is also found that β-PCNTs of the smallest/biggest size consist of 12/44 atoms. According to electronic band structure calculations, β-PCNTs can be semiconductors, semimetals or metals depending on their size and form (see Figure 1b). Therefore, due to their extraordinary form and highly tunable band structure, β-PCNTs may find the application in straintronic, optical and photovoltaic devices.
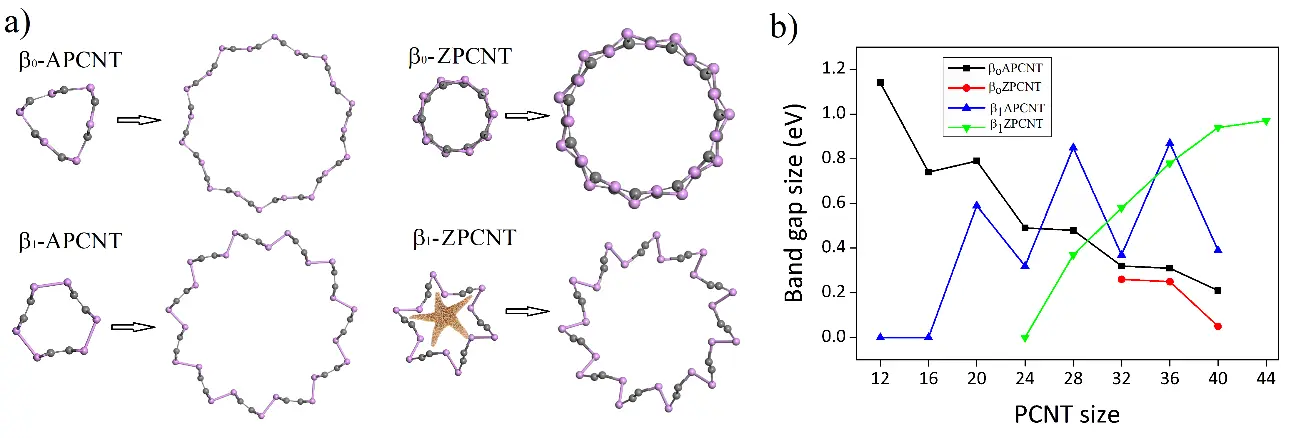
Figure 1.(a) Atomic structure and (b) band gap size as a function of size of β 0– and β 1-APCNT and β 0– and β 1-ZPCNT.
Acknowledgement.A.A.K. M.H. and W.C. acknowledges the financial support by the Academy of Finland (grant No. 311934). S.A.Sh. acknowledges the financial support by the Ministry of Education and Science of the Russian Federation (state task in the field of scientific activity, Southern Federal University), theme N BAS0110/20-3-08IF.
References:
[1] X. Huang, Y. Cai, X. Feng, W. C. Tan, D. Md. N. Hasan, L. Chen, N. Chen, L. Wang, L. Huang, T. J. Duffin, C. A. Nijhuis, Y. W. Zhang, C. Lee and K. W. Ang, ACS Photonics, 5(8), 3116–3123 (2018)
[2] S. A. Shcherbinin, K. Zhou, S. V. Dmitriev, E. A. Korznikova, A. R. Davletshin and A. A. Kistanov, J. Phys. Chem. C, 124(18), 10235-10243 (2020)
Computational search for new high-T Csuperconductors with subsequent synthesis
A.G. Kvashnin 1, I.A. Kruglov 2,3, D.V. Semenok 1, A.R. Oganov 1,
1 – Skolkovo Institute of Science and Technology, Moscow, Russia
2 – Moscow Institute of Physics and Technology, Dolgoprudny, Russia
3 – Dukhov Research Institute of Automatics (VNIIA), Moscow, Russia
A.Kvashnin@skoltech.ru
Hydrogen-rich hydrides attract great attention due to recent theoretical [1] and then experimental discovery of record high-temperature superconductivity in H 3S (T C= 203 K at 155 GPa [2]).
Here we perform a systematic evolutionary search for new phases in the Fe-H [3], Th-H [4], U-H [5] and other numerous systems under pressure [6] in order to predict new materials which are unique high-temperature superconductors.
We predict new hydride phases at various pressures using the variable-composition search as implemented in evolutionary algorithm USPEX [7–9]. Among the Fe-H system two potentially high-T CFeH 5and FeH 6phases in the pressure range from 150 to 300 GPa were predicted and were found to be superconducting within Bardeen-Cooper-Schrieffer theory, with T Cvalues of up to 46 K. Several new thorium hydrides were predicted to be stable under pressure using evolutionary algorithm USPEX, including ThH 3, Th 3H 10, ThH 4, ThH 6, ThH 7and ThH 10. Fcc -ThH 10was found to be the highest-temperature superconductor with T Cin the range 221–305 K at 100 GPa. Actinide hydrides show, i.e. AcH 16was predicted to be stable at 110 GPa with T Cof 241 K.
To continue this theoretical study, we performed an experimental synthesis of Th-H phases at high-pressures including ThH 10. Obteined results can be found in Ref. [10].
Acknowledgement.This work was supported by RFBR foundation № 19-03-00100 and facie foundation, grant UMNIK № 13408GU/2018.
References:
[1] D. Duan et al., Sci. Rep . 4, 6968 (2018)
[2] A.P. Drozdov et al. Nature . 525, 73–76 (2015)
[3] A.G. Kvashnin at al. J. Phys. Chem. C, 122 4731–4736 (2018)
[4] A.G. Kvashnin et al. ACS Applied Materials & Interfaces 10, 43809-43816 (2018)
[5] I.A. Kruglov et al. Sci. Adv. 4, eaat9776. (2018)
[6] D.V. Semenok et al. J. Phys. Chem. Lett . 8, 1920–1926 (2018)
[7] A.O. Lyakhov et al. Comp. Phys. Comm . 184, 1172–1182 (2013)
[8] A.R. Oganov et al. J. Chem. Phys . 124, 244704 (2006)
[9] A.R. Oganov et al. Acc. Chem. Res . 44 227–237 (2011)
[10] D.V. Semenok et al. Mat. Today., 33, 36–44 (2020)

Enhanced Electrocatalytic Activities by Substitutional Tuning of Nickel-based Ruddlesden-Popper Catalysts for the Oxidation of Urea and Small Alcohols
Stevenson, K. J. 1
1 – Skolkovo Institute of Science and Technology, Moscow, Russia
k.stevenson@skoltech.ru
The electrooxidation of urea continues to attract considerable interest as an alternative to the oxygen evolution reaction (OER) as the anodic reaction in the electrochemical generation of hydrogen due to the lower potential required to drive the reaction and the abundance of urea available in waste streams. In this talk the effect of Sr substitution in a series of La 2-xSrxNiO 4+δRuddlesden-Popper catalysts on the electrooxidations of urea, methanol, and ethanol are presented. We demonstrate that activities toward the urea oxidation reaction increase with increasing Ni oxidation state. The 75 % Sr-substituted La 0.5Sr 1.5NiO 4+δcatalyst exhibits a mass activity of 588 mA 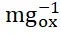
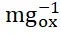 and 7.85 A
and 7.85 A 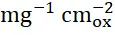
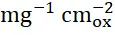 for the electrooxidation of urea in 1 M KOH containing 0.33 M urea, demonstrating the potential applications of Ni-based Ruddlesden-Popper materials for direct urea fuel cells and low-cost hydrogen production.[1] Additionally, we find the same correlations between Ni oxidation state and activities for the electrooxidations of methanol and ethanol, as well as identify processes that result in catalyst deactivation for all three oxidations. This demonstration of how systematically increasing Ni – O bond covalency by raising the formal oxidation state of Ni above +3 serves to increase catalyst activity for these reactions acts as a governing principle for the rational design of catalysts for the electrooxidation of urea and other small molecules going forward [2]
for the electrooxidation of urea in 1 M KOH containing 0.33 M urea, demonstrating the potential applications of Ni-based Ruddlesden-Popper materials for direct urea fuel cells and low-cost hydrogen production.[1] Additionally, we find the same correlations between Ni oxidation state and activities for the electrooxidations of methanol and ethanol, as well as identify processes that result in catalyst deactivation for all three oxidations. This demonstration of how systematically increasing Ni – O bond covalency by raising the formal oxidation state of Ni above +3 serves to increase catalyst activity for these reactions acts as a governing principle for the rational design of catalysts for the electrooxidation of urea and other small molecules going forward [2]
Читать дальше