More crowded than ever, the laboratory was showing signs of wear and tear. Mark Oliphant remembers standing in the hallway outside Rutherford’s office for the first time and noticing “uncarpeted floor boards, dingy varnished pine doors and stained plastered walls, indifferently lit by a skylight with dirty glass.” 504Oliphant also records Rutherford’s appearance at that time, the late 1920s, when the Cavendish director was in his midfifties: “I was received genially by a large, rather florid man, with thinning fair hair and a large moustache, who reminded me forcibly of the keeper of the general store and post office in a little village in the hills behind Adelaide where I had spent part of my childhood. Rutherford made me feel welcome and at ease at once. He spluttered a little as he talked, from time to time holding a match to a pipe which produced smoke and ash like a volcano.”
With simple experimental apparatus Rutherford continued to produce astonishing discoveries. The most important of them besides the discovery of the nucleus had come to fruition in 1919, shortly before he left Manchester for Cambridge—he sent off the paper in April. Afterward, at the Cavendish, he and James Chadwick followed through. The 1919 Manchester paper actually summarized a series of investigations Rutherford carried out in his rare moments of spare time during the four years of war, when he kept the Manchester lab going almost singlehandedly while doing research for the Admiralty on submarine detection. It appeared in four parts. The first three parts cleared the way for the fourth, “An anomalous effect in nitrogen,” which was revolutionary. 505
Ernest Marsden, whose examination of alpha scattering had led Rutherford to discover the atomic nucleus, had found a similarly fruitful oddity in the course of routine experimental studies at Manchester in 1915. Marsden was using alpha particles—helium nuclei, atomic weight 4—emanating from a small glass tube of radon gas to bombard hydrogen atoms. He did that by fixing the radon tube inside a sealed brass box fitted at one end with a zinc-sulfide scintillation screen, evacuating the box of air and then filling it with hydrogen gas. The alpha particles emanating from the radon bounced off the hydrogen atoms (atomic weight approximately 1) like marbles, transferring energy to the H atoms and setting some of them in motion toward the scintillation screen; Marsden then measured their range by interposing pieces of absorbing metal foils behind the screen until the scintillations stopped. Predictably, the less massive H atoms recoiled farther as a result of their collisions with the heavier alpha particles than did the alphas—about four times as far, says Rutherford—just as smaller and larger marbles colliding in a marbles game do.
That was straightforward enough. But then Marsden noticed, Rutherford relates, while the box was evacuated, that the glass radon tube itself “gave rise to a number of scintillations like those from hydrogen.” He tried a tube made of quartz, then a nickel disk coated with a radium compound, and found similarly bright, H-like scintillations. “Marsden concluded that there was strong evidence that hydrogen arose from the radioactive matter itself.” 506This conjecture would have been stunning, if true—so far radioactive atoms had been found to eject only helium nuclei, beta electrons and gamma rays in the course of their decay—but it was not the only possible deduction. Nor was it one that Rutherford, who after all had discovered two of the three basic radiations and had never found hydrogen among them, was likely to accept out of hand. Marsden had returned to New Zealand in 1915 to teach; Rutherford pursued the strange anomaly. He had a good idea what he was after. “I occasionally find an odd half day to try a few of my own experiments,” he wrote Bohr on December 9, 1917, “and have got I think results that will ultimately prove of great importance. I wish you were here to talk matters over with. I am detecting and counting the lighter atoms set in motion by [alpha] particles…. I am also trying to break up the atom by this method.” 507
His equipment was similar to Marsden’s, a small brass box fitted with stopcocks to admit and evacuate gases from its interior, with a scintillation screen mounted on one end. For an alpha source he used a beveled brass disk coated with a radium compound:
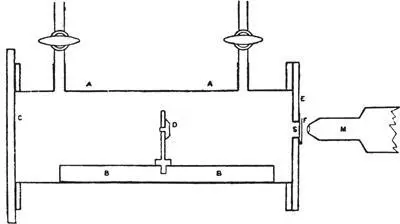
Arrangement of Ernest Rutherford’s experiment: D, alpha source. S, zinc sulfide scintillation screen. M, microscope.
The likeliest explanation for Marsden’s anomalous H atoms was contamination; hydrogen is light and chemically active and a minor component of the ubiquitous air. So Rutherford’s problem was basically one of rigorous exclusion. He needed to narrow down the possible sources of hydrogen atoms in his box until he could conclusively prove their point of origin. He started by showing that they did not come from the radioactive materials alone. He established that they had the same mass and expected range as the H atoms that recoiled from alpha bombardment of hydrogen gas in Marsden’s experiment. He admitted dry oxygen into the evacuated brass box, then carbon dioxide, and found in both cases that the H atoms coming off the radioactive source were slowed down by colliding with the atoms of those gases—fewer scintillations showed up on the screen.
Then he tried dry air. The result surprised him. Instead of decreasing the number of scintillations, as oxygen and carbon dioxide had done, dry air increased them— doubled them in fact.
These newfound scintillations “appeared to the eye to be about equal in brightness to H scintillations,” Rutherford notes cautiously near the beginning of the revolutionary Part IV of his paper. 508He went after them. If they were H atoms, they still might be contaminants. He eliminated that possibility first. He showed that they could not be due merely to the hydrogen in water vapor (H 2O): drying the air even more thoroughly made little difference in their number. Dust might harbor H atoms like dangerous germs: he filtered the air he let into the box through long plugs of absorbent cotton but found little change.
Since the increase in H atoms occurred in air but not in oxygen or carbon dioxide, Rutherford deduced then that it “must be due either to nitrogen or to one of the other gases present in atmospheric air.” And since air is 78 percent nitrogen, that gas appeared to be the likeliest candidate. He tested it simply, by comparing scintillations from air to scintillations from pure nitrogen. The test confirmed his hunch: “With pure nitrogen, the number of long-range scintillations under similar conditions was greater than in air.” 509Finally, Rutherford established that the H atoms came in fact from the nitrogen and not from the radioactive source alone. And then he made his stunning announcement, couching it as always in the measured understatement of British science: “From the results so far obtained it is difficult to avoid the conclusion that the long-range atoms arising from collision of [alpha] particles with nitrogen are not nitrogen atoms but probably atoms of hydrogen…. If this be the case, we must conclude that the nitrogen atom is disintegrated.” 510Newspapers soon published the discovery in plainer words: Sir Ernest Rutherford, headlines blared in 1919, had split the atom.
It was less a split than a transmutation, the first artificial transmutation ever achieved. When an alpha particle, atomic weight 4, collided with a nitrogen atom, atomic weight 14, knocking out a hydrogen nucleus (which Rutherford would shortly propose calling a proton), the net result was a new atom of oxygen in the form of the oxygen isotope 017: 4 plus 14 minus 1. There would hardly be enough 017 to breathe; only about one alpha particle in 300,000 crashed through the electrical barrier around the nitrogen nucleus to do its alchemical work. 511
Читать дальше













