Geiger went to work on alpha scattering, aided by Ernest Marsden, then an eighteen-year-old Manchester undergraduate. They observed alpha particles coming out of a firing tube and passing through foils of such metals as aluminum, silver, gold and platinum. The results were generally consistent with expectation: alpha particles might very well accumulate as much as two degrees of total deflection bouncing around among atoms of the plum-pudding sort. But the experiment was troubled with stray particles. 172Geiger and Marsden thought molecules in the walls of the firing tube might be scattering them. They tried eliminating the strays by narrowing and defining the end of the firing tube with a series of graduated metal washers. That proved no help.
Rutherford wandered into the room. The three men talked over the problem. Something about it alerted Rutherford’s intuition for promising side effects. Almost as an afterthought he turned to Marsden and said, “See if you can get some effect of alpha particles directly reflected from a metal surface.” Marsden knew that a negative result was expected—alpha particles shot through thin foils, they did not bounce back from them—but that missing a positive result would be an unforgivable sin. 173He took great care to prepare a strong alpha source. He aimed the pencil-narrow beam of alphas at a forty-five degree angle onto a sheet of gold foil. He positioned his scintillation screen on the same side of the foil, beside the alpha beam, so that a particle bouncing back would strike the screen and register as a scintillation. Between firing tube and screen he interposed a thick lead plate so that no direct alpha particles could interfere.
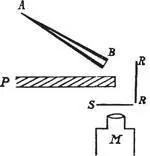
Arrangement of Ernest Marsden’s experiment: A-B, alpha particle source. R-R, gold foil. P, lead plate. S, zinc sulfide scintillation screen. M, microscope.
Immediately, and to his surprise, he found what he was looking for. “I remember well reporting the result to Rutherford,” he wrote, “…when I met him on the steps leading to his private room, and the joy with which I told him.” 174
A few weeks later, at Rutherford’s direction, Geiger and Marsden formulated the experiment for publication. “If the high velocity and mass of the α-particle be taken into account,” they concluded, “it seems surprising that some of the α-particles, as the experiment shows, can be turned within a layer of 6 × 10 −5[i.e., .00006] cm. of gold through an angle of 90°, and even more. To produce a similar effect by magnetic field, the enormous field of 10 9absolute units would be required.” Rutherford in the meantime went off to ponder what the scattering meant. 175
He pondered, in the midst of other work, for more than a year. He had a first quick intuition of what the experiment portended and then lost it. 176Even after he announced his spectacular conclusion he was reluctant to promote it. One reason for his reluctance might be that the discovery contradicted the atomic models J. J. Thomson and Lord Kelvin had postulated earlier. There were physical objections to his interpretation of Marsden’s discovery that would require working out as well.
Rutherford had been genuinely astonished by Marsden’s results. “It was quite the most incredible event that has ever happened to me in my life,” he said later. “It was almost as incredible as if you fired a 15-inch shell at a piece of tissue paper and it came back and hit you. On consideration I realised that this scattering backwards must be the result of a single collision, and when I made calculations I saw that it was impossible to get anything of that order of magnitude unless you took a system in which the greatest part of the mass of the atom was concentrated in a minute nucleus.” 177
“Collision” is misleading. What Rutherford had visualized, making calculations and drawing diagrammatic atoms on large sheets of good paper, was exactly the sort of curving path toward and away from a compact, massive central body that a comet follows in its gravitational pas de deux with the sun. 178He had a model made, a heavy electromagnet suspended as a pendulum on thirty feet of wire that grazed the face of another electromagnet set on a table. 179With the two grazing faces matched in polarity and therefore repelling each other, the pendulum was deflected into a parabolic path according to its velocity and angle of approach, just as the alpha particles were deflected. He needed as always to visualize his work.
When further experiment confirmed his theory that the atom had a small, massive nucleus, he was finally ready to go public. He chose as his forum an old Manchester organization, the Manchester Literary and Philosophical Society—“largely the general public,” says James Chadwick, who attended the historic occasion as a student on March 7, 1911, “…people interested in literary and philosophical ideas, largely business people.” 180
The first item on the agenda was a Manchester fruit importer’s report that he had found a rare snake in a consignment of Jamaica bananas. 181He exhibited the snake. Then it was Rutherford’s turn. Only an abstract of the announcement survives, but Chadwick remembers how it felt to hear it: it was “a most shattering performance to us, young boys that we were…. We realized this was obviously the truth, this was it.” 182
Rutherford had found the nucleus of his atom. He did not yet have an arrangement for its electrons. At the Manchester meeting he spoke of “a central electric charge concentrated at a point and surrounded by a uniform spherical distribution of opposite electricity equal in amount.” That was sufficiently idealized for calculation, but it neglected the significant physical fact that the “opposite electricity” must be embodied in electrons. 183Somehow they would have to be arranged around the nucleus.
Another mystery. A Japanese theoretical physicist, Hantaro Nagaoka, had postulated in 1903 a “Saturnian” model of the atom with flat rings of electrons revolving like Saturn’s rings around a “positively charged particle.” 184Nagaoka adapted the mathematics for his model from James Clerk Maxwell’s first triumphant paper, published in 1859, “On the stability of motion of Saturn’s rings.” All Rutherford’s biographers agree that Rutherford was unaware of Nagaoka’s paper until March 11, 1911—after the Manchester meeting—when he heard about it by postcard from a physicist friend: “Campbell tells me that Nagaoka once tried to deduce a big positive centre in his atom in order to account for optical effects.” He thereupon looked up the paper in the Philosophical Magazine and added a discussion of it to the last page of the full-length paper, “The scattering of a and β particles by matter and the structure of the atom,” that he sent to the same magazine in April. 185He described Nagaoka’s atom in that paper as being “supposed to consist of a central attracting mass surrounded by rings of rotating electrons.” 186
But it seems that Nagaoka had recently visited him, because the Japanese physicist wrote from Tokyo on February 22, 1911, thanking him “for the great kindness you showed me in Manchester.” [1] Nagaoka indicates indirectly that the visit took place sometime prior to July 1910—after Marsden’s 1909 discovery and before Rutherford’s announcement to Geiger at Christmastime 1910 that he had worked out an explanation.
Yet the two physicists seem not to have discussed atomic models, or Nagaoka would probably have continued the discussion in his letter and Rutherford, a totally honest man, would certainly have acknowledged it in his paper. 187
Читать дальше













