This image of four spectra is taken from one of Huggins's publications. These are wavelengths of light in the visible part of the spectrum to which the eye is sensitive. At the bottom is the spectrum of an 1868 comet called Brorsen. Above that is the spectrum of another 1868 comet called Winnecke II. And at the top is the spectrum of olive oil.
You can see that Comet Winnecke resembles olive oil more than it does Comet Brorsen. However, nobody deduced the existence of olive oil on the comets. (It would be an important discovery if it could be made.) But instead what this similarity shows is that a molecular fragment, diatomic carbon or C 2-two carbon atoms attached together-is present when you look at the spectrum of the comets and also when you look at natural gas and the vapor from heated olive oil. This is the discovery of an organic molecule, not one very familiar on Earth because of its instability when it collides 'with other molecules. It requires something close to a high vacuum, which does not naturally occur on the surface of the Earth. In the vicinity of a cometary coma, there is a high vacuum sufficient for C 2not to be destroyed, and so here it is-the first discovery of an extraterrestrial organic molecule. And it turns out not to be one with which we have great familiarity.
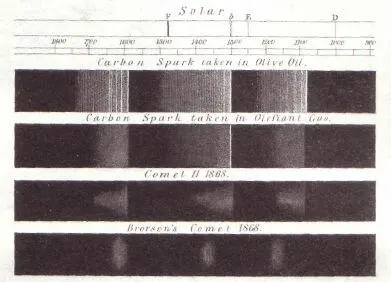
fig. 21
Spectrum of Comet 2001 Q4 (NEAT) on 2004 May 14
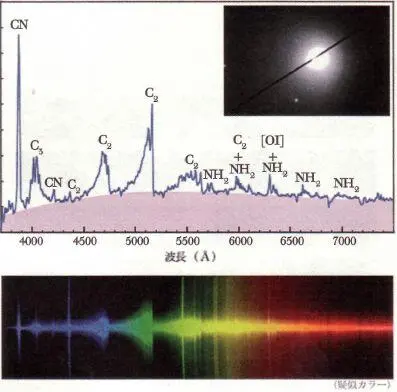
fig. 22
Here is a typical modern cometary spectrum, and we can see the prominent bands of C 2and other things, too. We see NH, the amino group that is produced by dissociation of ammonia, NH, which is also the defining molecular group of the amino acids, the building blocks of proteins. And we see here the molecular fragment that caused all the trouble, CN, the nitrile or cyanide molecule.
A single grain of potassium cyanide on the tongue will instantly kill a human being. Discovering cyanide in comets worried people.
Especially when it appeared that in 1910 the Earth would pass through the tail of Halley's Comet. Astronomers tried to reassure people. They said it wasn't clear that the Earth would pass through the tail, and even if the Earth did pass through the tail, the density of CN molecules was so low that it 'would be perfectly all right. But nobody believed the astronomers.
Perhaps the Earth did pass through the edge of the tail. In any case the comet came and went, nobody died, and in fact nobody could detect a single additional molecule of CN anywhere on the Earth. William Huggins, however, did die at the time that the comet came by, but not of cyanide poisoning.
Now, when we look closely at a comet, there is a tiny nucleus, the solid body that constitutes the comet everywhere except when it's very close to the Sun. The icy nucleus is typically a few kilometers across-but when it comes close to the Sun, the icy nucleus outgasses mainly water vapor and produces the coma and a long and lovely tail.
Consider the molecules we have just talked about: CN, C 2, C, NH. What are their parent molecules? Where did they come from? There are some precursors. We are seeing only fragments that have been chopped off of a bigger molecule by ultraviolet light from the Sun and the solar wind. It is clear that there is a repository of much more complex molecules-much more complex organic molecules-that are part of the cometary nucleus but which we have not yet discovered.
Radio astronomical studies have already found HCN (hydrogen cyanide) and CH 3CH (acetonitrile) in at least one comet. And these are interesting organic molecules that in other ways are implicated in the origin of life on Earth.
Imagine the air in front of your nose, highly magnified, say 10 million times. You would see a multitude of molecules, nitrogen and oxygen molecules, and occasional molecules of water

fig. 23
vapor and carbon dioxide. Air, as you know, is mainly oxygen and nitrogen. Now, if you take some air and cool it, you will progressively condense out the various molecules. Water will condense out first, carbon dioxide next, oxygen and nitrogen much later; that is, at much lower temperatures.
Let's consider the condensation of the water molecule. When condensation happens, it's not just that the water molecules drop out of the air helter-skelter. In fact they form a lovely hexagonal crystal lattice, which stretches off as far as the ice crystal or snowflake or whatever it is goes. Other molecules condense out at much higher temperatures, like silica, for example (silicon dioxide), which also forms a crystal lattice.
Let's go back to the solar nebula from which, as we said earlier, the solar system almost surely formed, with a protosun in the center and the temperature declining the farther we get from the Sun. Now we must imagine this as a mix of cosmically abundant materials, including water (H 20, which we know through spectroscopic analysis of astronomical images is very abundant), methane (CH 4; we know that's very abundant), silica (Si0 2; we know that's very abundant), and what happens is that at different distances from the Sun, different materials will condense out, because they have different vapor pressures or different melting points. And what we see is (guess what?), water condenses out roughly at the vicinity of the Earth, whereas silicates condense out closer to the Sun, so liquid silicates or gaseous silicates are not to be expected under ordinary planetary conditions, even at the orbit of Mercury Whereas you have to go out to somewhere near the present distance of Saturn before methane condenses. Now, methane is probably the chief carbon-containing molecule in the cosmos, and what this says is that in the early stages of the formation of the solar nebula there should have been a preferential condensation of methane in the outer parts of the solar system, but not in the inner parts. And if that is generally true, then we ought to expect more organic matter in the outer parts and much less in our neck of the cosmic woods.
Well, there is certainly not a huge amount of methane on the Moon or Mercury. But when we do go out to the orbit of Saturn we start finding not only evidence for methane-the planets Jupiter, Saturn, Uranus, and Neptune have lots of methane in their spectra-but we find a set of data that strongly implies the presence of complex organic molecules in the outer solar system.
This is a photograph of Iapetus, one of the outer moons of Saturn. The gray area is not in shadow. There is actually a remarkable division of one hemispheric surface into dark material and the other hemisphere into bright material. And the clear spectral signature of water ice is present in the bright areas.
We did not fly very close with either Voyager 1 or Voyager 2 to Iapetus. We think this is organic matter. It is very dark. At the center of this dark stuff, the albedo, the reflectivity, is something like 5 percent. I can't be sure, but I suspect that there is nothing in the room you are sitting in as dark as 5 percent albedo. Also, it is reddish. That is, it does not reflect much light, but it reflects more light in the red than in the blue part of the visible spectrum. And the values of the albedo and color are inconsistent with a wide range of other materials that you might offhand guess it might be-various of the salts, for example. They are very consistent with complex organic matter of various sorts. We know there is complex organic matter out there. I gave you one argument from the comets. Another argument is a category of meteorites called carbonaceous meteorites that fall to Earth, and they have several percent to as much as 10 percent of complex organic matter in them.
Читать дальше













