9 . Give English equivalents to the following word combinations and learn them by heart:
сопротивляться коррозионному воздействию среды
металлы и сплавы
коррозионное разрушение поверхности
объем разрушенного металла
расплавы электролитов
действие радиоактивного излучения
жизнедеятельность микроорганизмов
радиационная коррозия
10 . Give Russian equivalents to the following word combinations and learn them by heart:
chloride ions
oxygen concentration
electrochemical reactions
wall thickness of a metal
wet corrosion
corrosion resistance
nonelectrolyte
ground corrosion
11 . Translate the following sentences into Russian.
1. Uniform corrosion is considered the form of corrosion that can be tolerated in marine structures and equipment.
2. Crevice corrosion occurs at places with gaskets, bolts and lap joints where crevice exists.
3. Pitting corrosion can be prevented through proper selection of materials with known resistance to the service environment.
4. The reaction starts at the surface and proceeds uniformly.
5. The gap or crevice can be formed between two metals or a metal and non-metallic material.
6. The most severe attack occurs at the joint between the two dissimilar metals.
7. Copper produces a green film of corrosion product containing hydrated sulphate, or carbonate, or chloride.
8. Pipelines or tanks that are exposed to an electrolyte with a low oxygen concentration are generally anodic to the same material exposed to an electrolyte with a high oxygen content.
9. Localized corrosion can be defined as selective removal of metal by corrosion at small special areas or zones on a metal surface in contact with a liquid environment.
10. The process of corrosion requires four elements: an anode, a cathode, an electrolyte, and a metallic path.
12 . Translate the following sentences into English.
1. Коррозия металлических сооружений наносит большой материальный и экономический ущерб.
2. Коррозия металлов бывает сплошной и местной.
3. Человечество несет огромные материальные потери в результате коррозии трубопроводов, деталей машин, судов, мостов, морских конструкций и технологического оборудования.
4. Наибольшую чувствительность к щелевой коррозии проявляют пассивные металлы в случае возможной их депассивации в щелях.
5. Наиболее распространенным и практически важным видом химической коррозии является газовая коррозия – коррозия металлов в сухих газах (воздух, продукты сгорания топлива) при высоких температурах.
6. Процессы окисления металла и восстановления окислительного компонента протекают раздельно в среде жидкого электролита.
7. Язвенная коррозия часто наблюдается у нержавеющих стальных сплавов.
8. Если водород не выделяется, происходит восстановление кислорода, и здесь говорят о кислородной коррозии, или коррозии с кислородной деполяризацией.
9. Коррозионное сопротивление нержавеющей стали зависит от тонкого невидимого пассивного слоя на стальной поверхности.
10. Этот слой состоит главным образом из хромированной окиси, которая формируется в реакции с кислородом содержащимся в воздухе.
13 . Translate the following word combinations.
Uniform corrosion – гальваническая коррозия – crevice corrosion – концентрационная коррозия – graphitic corrosion – подземная коррозия – electrolyte – окисление – corrosion resistance – медь – metal surface – коррозийное воздействие – oxygen concentration – распространение – gasket – процессы окисления – oxidizing component – сухой газ.
14 . Give the definitions of the words and word combinations.
1. Depolarization is …
2. Passive metals are …
3. Metallography is …
4. Liquid is …
5. Temperature is …
15 . Give the example(s) of the uniform corrosion.
16 . Answer the following questions using texts from Unit 2.
1. What types of corrosion do you know?
2. Give a definition of ground corrosion.
3. What is corrosion resistance?
4. How can artificial crevices be created?
17 . Summarize the general ideas developed in all the texts of the Unit 2.
Unit 3
MICROBIOLOGICAL CORROSION
1 . Read the international words, guess their meanings and give the Russian equivalents.
Microbiological, structural, industrial, process, microbes, biological, organism, aqueous, potential, transport, role, bacteria, anaerobic, aerobic, typical, thiobacillus, localize.
2 . Read and translate the following verbs.
To transport, to ensue, to cause, to occur, to tend, to determine, to deliver, to present, to decrease, to increase, to manifest, to settle, to be based, to engender, to reduce, to create, to operate, to perform, to penetrate, to consume, to thrive.
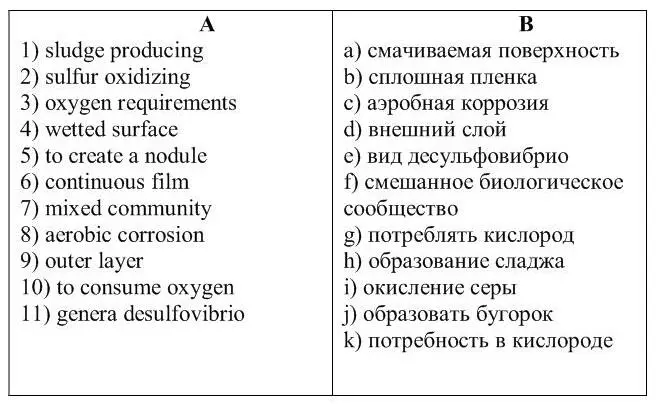
3 . Match the English phrases from column A with the Russian phrases in column B.
4. Read and translate the text. Summarize it in Russian.
Microbiological corrosion (MIC) refers to corrosion and ensuing loss of metal caused by biological organisms. MIC can occur in any aqueous environments and because of the present nature of microbes in fluid systems. MIC is a commonly occurring phenomenon. MIC is a common problem in industrial processes due to the presence of microbes, adequate nutrients and corrosive byproducts.
A number of metals, such as structural steels, copper alloys etc., tend to corrode generally over the entire surface in the absence of crevices or galvanic effects. In such cases, corrosion is determinated by the rate at which dissolved oxygen can be delivered to the metal surface. Biological organisms present in the aqueous medium often have the potential to increase or decrease oxygen transport to the surface; consequently, these organisms have a role in increasing or decreasing general corrosion. Most MIC, however, manifests as localized corrosion because most organisms do not form in a continuous film on the metal surface. Microscopic organisms also tend to settle on metal surfaces in the form of discrete colonies or at least spotty, rather than continuous films. Biological organisms fall under two groups based on the type of corrosion they engender: (a) anaerobic corrosion (b) aerobic corrosion. Sulfate reducing bacteria (SRB) from the genera desulfovibrio are a typical example of anaerobic MIC. Aerobic sulfur oxidizing bacteria of the type thiobacillus can create an environment of up to 10 percent sulfuric acid, thereby encouraging rapid corrosion.
Читать дальше
Конец ознакомительного отрывка
Купить книгу












