Various - The Atlantic Monthly, Volume 06, No. 33, July, 1860
Здесь есть возможность читать онлайн «Various - The Atlantic Monthly, Volume 06, No. 33, July, 1860» — ознакомительный отрывок электронной книги совершенно бесплатно, а после прочтения отрывка купить полную версию. В некоторых случаях можно слушать аудио, скачать через торрент в формате fb2 и присутствует краткое содержание. Жанр: foreign_antique, periodic, foreign_edu, на английском языке. Описание произведения, (предисловие) а так же отзывы посетителей доступны на портале библиотеки ЛибКат.
- Название:The Atlantic Monthly, Volume 06, No. 33, July, 1860
- Автор:
- Жанр:
- Год:неизвестен
- ISBN:нет данных
- Рейтинг книги:4 / 5. Голосов: 1
-
Избранное:Добавить в избранное
- Отзывы:
-
Ваша оценка:
- 80
- 1
- 2
- 3
- 4
- 5
The Atlantic Monthly, Volume 06, No. 33, July, 1860: краткое содержание, описание и аннотация
Предлагаем к чтению аннотацию, описание, краткое содержание или предисловие (зависит от того, что написал сам автор книги «The Atlantic Monthly, Volume 06, No. 33, July, 1860»). Если вы не нашли необходимую информацию о книге — напишите в комментариях, мы постараемся отыскать её.
The Atlantic Monthly, Volume 06, No. 33, July, 1860 — читать онлайн ознакомительный отрывок
Ниже представлен текст книги, разбитый по страницам. Система сохранения места последней прочитанной страницы, позволяет с удобством читать онлайн бесплатно книгу «The Atlantic Monthly, Volume 06, No. 33, July, 1860», без необходимости каждый раз заново искать на чём Вы остановились. Поставьте закладку, и сможете в любой момент перейти на страницу, на которой закончили чтение.
Интервал:
Закладка:
Various
The Atlantic Monthly, Volume 06, No. 33, July, 1860 / A Magazine Of Literature, Art, And Politics
METEOROLOGY
A GLANCE AT THE SCIENCE
The purpose of this article is to present, in a brief and simple manner, the leading principles on which the science of Meteorology is founded,–rather, however, in the spirit of an inquirer than of a teacher. For, notwithstanding the rapid progress it has made within the last thirty years, it is far from having the authority of an exact science; many of its phenomena are as yet inexplicable, and many differences of opinion among the learned remain unreconciled on points at first sight apparently easy to be settled.
Meteorology has advanced very far beyond its original limits. Spherical vapor and atmospheric space give but a faint idea of its range. We find it a leading science in Physics, and having intimate relations with heat, light, electricity, magnetism, winds, water, vegetation, geological changes, optical effects, pneumatics, geography,–and with climate, controlling the pursuits and affecting the character of the human race. It is so intimately blended, indeed, with the other matters here named, as scarcely to have any positive boundary of its own; and its vista seems ever lengthening, as we proceed.
Without dwelling upon the numerous consequences which flow from meteorological influences, let us see what is properly included under the subject of Meteorology. And first, of the Atmosphere.
This is a gaseous, vapor-bearing, elastic fluid, surrounding the earth. Its volume is estimated at 1/29th, and its weight at about 43/1000ths, that of the globe. It is composed of 21 parts in weight of Oxygen and 77 of Nitrogen, with a little Carbonic Acid, Aqueous Vapor, and a trace of Carburetted Hydrogen. There are numerous well-known calculations of the proportions of the various constituents of the atmosphere, which we owe to Priestley, Dalton, Black, Cavendish, Liebig, and others; but that given by Professor Ansted is sufficiently simple and intelligible. In 10 volumes or parts of it, he gives to
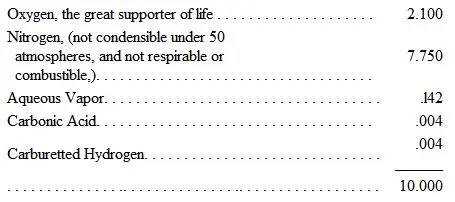
and he adds a trace of Ammoniacal Vapor. It is usual to state the proportions of air as being 1 Oxygen to 4 Nitrogen.
It is a curious fact, that, while there are six varieties of compounds of nitrogen and oxygen, but one of these is fitted to sustain life, and that is our atmosphere.
It is well enough to note, that, when we use the word volume or measure, in speaking of the atmosphere or any gaseous body, we adopt the theory of Gay- Lussac, who discovered that gases unite with each other in definite proportions whenever they enter into combination. This theory led to important results; for by knowing the elements of a compound gas, we easily determine its specific gravity.
It has been attempted to apply the principle to organic bodies; but it has not yet been carried to a full and satisfactory conclusion. It may be noticed, too, that Dalton affirmed that simple substances unite with each other in definite weights to form compound substances, thus supporting the idea of Lussac. These discoveries were made about the same time, Dalton having the credit of originating them. Various modifications of the principle have been from time to time presented to public attention.
Whether the constituents of the atmosphere are chemically or mechanically combined,–one of the things about which the learned are not fully agreed,–it is found to be chemically the same in its constituents, all over the world, whether collected on mountains or on plains, on the sea or on the land, whether obtained by aëronauts miles above the earth or by miners in their deepest excavations. On the theory of its mechanical combination, however, as by volume, and that each constituent acts freely for itself and according to its own laws, important speculations (conclusions, indeed) have arisen, both as regards temperature and climatic differences. It should be observed, that volume, as we have used the word, is the apparent space occupied, and differs from mass, which is the effective space occupied, or the real bulk of matter, while density is the relation of mass to volume, or the quotient resulting from the division of the one by the other. Those empty spaces which render the volume larger than the mass are technically called its pores.
Has the composition of the atmosphere changed in the lapse of years? On this point both French and German philosophers have largely speculated. It is computed that it contains about two millions of cubic geographical miles of oxygen, and that 12,500 cubic geographical miles of carbonic acid have been breathed out into the air or otherwise given out in the course of five thousand years. The inference, then, should be, that the latter exists in the air in the proportion of 1 to 160, whereas we find but 4 parts in 10,000. Dumas and Bossingault decided that no change had taken place, verifying their conclusion by experiments founded on observations for more than thirty-five years. No chemical combination of oxygen and nitrogen has ever been detected in the atmosphere, and it is presumed none will be.
The atmosphere possesses, as may be readily imagined, many important characteristics. One of these is Weight.
This is demonstrated by simple, yet decisive experiments. The discovery of the fact is attributed to the illustrious Galileo, but to modern science we owe all the certainty, variety, and elegance of the demonstration. A vessel containing a quantity of air is weighed; the air is exhausted from it and it is weighed again. An accurate scale will then detect the difference of weight. A cubic foot of air weighs 1.2 oz. Hence a column of air of one inch in diameter and a mile in height weighs 44 oz.
The atmosphere is supposed to have an elevation of from 45 to 50 miles, but its weight diminishes in proportion to its height. The whole pressure at the surface of the earth is estimated to be 15 lbs. to the square inch; a person of ordinary size is consequently pressed upon by a weight of from 13 to 14 tons. Happily for us, the pressure from without is counteracted by the pressure from within.
The weight of the air is of great importance in the economy of Nature, since it prevents the excessive evaporation of the waters upon the earth's surface, and limits its extent by unalterable laws. Water boils at a certain temperature when at the earth's surface, where the weight of the atmosphere is greatest, but at different temperatures at different elevations from the surface. At the level of the sea it boils at 212°. On the high plains of Quito, 8,724 feet above the sea, it boils at 194°, and an egg cannot be cooked there in an open vessel. At Potosí the boiling-point is still lower, being 188°, and the barometrical column stands at 18°. Indeed, the experiment is often exhibited at our chemical lectures, of a flask containing a small quantity of water, which, exhausted of air, is made to boil by the ordinary heat of the hand.
Fahrenheit proposed to ascertain the height of mountains by this principle, and a simple apparatus was contrived for the purpose, which is now in successful use. The late Professor Forbes of Edinburgh, whose untimely death the friends of science have had so much reason to deplore, ascertained that the temperature of boiling water varied arithmetically with the height, and at the rate of one degree of the thermometric scale for every 549.05 feet. Multiplying the difference of the boiling-point by this number of feet, we have the elevation. The weight of the atmosphere, as indicated by the barometer, is also a means for ascertaining the height of mountains or of plains; but correction must be made for the effects of expansion or contraction, and for capillarity, or the attraction between the mercury and the glass tube, at least whenever great exactness is required. Tables for the convenience of calculation are given in several scientific works, and particularly in a paper of Professor Forbes, Ed. Trans. Vol. 15. Briefly, however, we may state, that between 0° and 32°, 34 thousandths of an inch must be allowed for depression or contraction, and between 32° and 52° 33 thousandths. The weight of the atmosphere is not only affected by rarefaction, but by currents of air, which give it a sudden density or rarity. Those who have ascended mountains have experienced both these changes.
Читать дальшеИнтервал:
Закладка:
Похожие книги на «The Atlantic Monthly, Volume 06, No. 33, July, 1860»
Представляем Вашему вниманию похожие книги на «The Atlantic Monthly, Volume 06, No. 33, July, 1860» списком для выбора. Мы отобрали схожую по названию и смыслу литературу в надежде предоставить читателям больше вариантов отыскать новые, интересные, ещё непрочитанные произведения.
Обсуждение, отзывы о книге «The Atlantic Monthly, Volume 06, No. 33, July, 1860» и просто собственные мнения читателей. Оставьте ваши комментарии, напишите, что Вы думаете о произведении, его смысле или главных героях. Укажите что конкретно понравилось, а что нет, и почему Вы так считаете.












