A special case is the preparation of catalyst nanoparticles on 3D macrostructures consisting of 2D graphene nanosheets with a foam‐like structure, namely, graphene aerogels. When GO is reduced under solvothermal mild conditions, the graphene nanosheets self‐assemble into 3D hydrogels, which can be dried subsequently by freeze‐drying or supercritical CO 2to produce graphene aerogels [14]. These 3D structures are favorable as structured catalytic reactors due to the ease in handling and separation. It is very interesting that the formation of both metal nanoparticles and the graphene aerogel support can take place in a one‐pot solvothermal synthesis using hot water as a mild reducing agent. The addition of divalent and trivalent ions (e.g. Ca 2+, Mg 2+, Cu 2+, Pb 2+, Cr 3+, Fe 3+) in GO dispersion promotes the formation of GO hydrogels [15]. Fe 2+ions are anchored onto the oxygenated functional groups of GO and hydrolyzed at acid pH to give Fe oxide nanorods (60 nm) or precipitated as oxide nanoparticles (30 nm) at basic pH [16]. By adding a reducing agent, 10 nm Fe oxide nanoparticles wrapped by graphene aerogels could be prepared [17]. By using also the one‐pot hydrothermal reduction method but adding some mild reductants such as sodium citrate and sodium acetate, the size of Fe 3O 4clusters was reduced to 5 nm on graphene aerogel [18]. Adding ascorbic acid as reductant and 100 °C solvothermal treatment, 5–13 nm noble metal nanoparticles have been prepared on graphene aerogels[19]. Instead of using aqueous dispersion of GO, a GO dispersion in ethylene glycol and subsequent hydrothermal method was used to prepare 4–6 nm Ru nanoparticles on graphene aerogel [20]. It is possible to decouple the formation of the rGO hydrogel and the deposition of the metal using a two‐step approach. By capitalizing on the advantage of high water content within the rGO hydrogel, an aqueous solution of the metal is infiltrated into the hydrogel. This approach has been used to introduce MoS 2in the hydrogel, which is subsequently freeze‐dried [21].
4.2.2.3 Graphene Derivatives: Doped Graphene and Synthetic Derivatives
Graphyne and graphdiyne are synthetic derivatives of graphene. They are synthetic flat single atomic layers consisting of carbon hexagons connected by linear carbon chains instead of only carbon hexagons as in graphene. In graphyne structure, the hexagons are bonded by linear acetylenic chains, whereas in graphdiyne the hexagons are bonded by two acetylenic chains ( Figure 4.2A–C). Graphdiyne is a new man‐made carbon allotrope prepared from a molecular precursor (hexaethynylbenzene) that possesses uniform 18 C‐hexagonal pores formed by three butadiyne linkages (‐C between the benzene rings), which can provide ideal anchoring sites for SACs with high stability as demonstrated by the results of theoretical calculations and in experiments in hydrogen evolution reaction (HER) [22]. The research about these graphene derivatives as catalyst support is almost unexplored due to the novelty of the material. It is foreseen that these derivatives are more amenable to functionalization with SACs than their parent material graphene. Therefore, a research field remains open for new researchers.
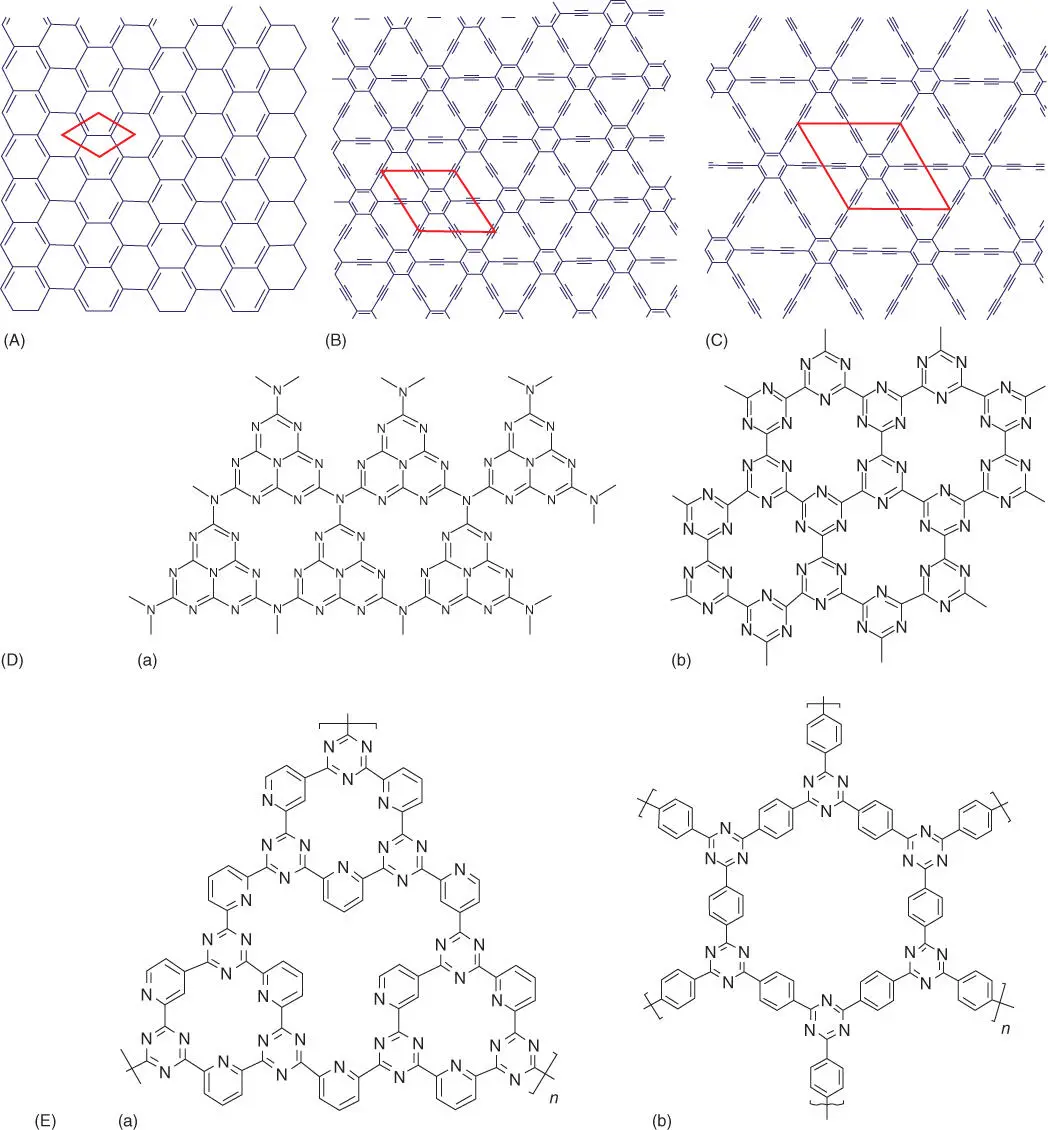
Figure 4.2 Structure of some 2D structures related to graphene: (A) graphene; (B, C) synthetic graphene derivatives (graphyne and graphdiyne); (D) graphitic carbon nitride, based on heptazine unit (a) and triazine unit (b); (E) covalent triazine frameworks (CTFs) with different structures: (a) CTF‐1 with hexagonal packing of pores synthesized by ionothermal trimerization and (b) PCTF‐1 synthesized by phosphorous pentoxide catalyst (P 2O 5) catalyzed condensation method.
4.2.3 Catalyst on Nanodiamonds and Onion‐Like Carbon
Nanodiamonds are diamonds (carbon allotrope with sp 3hybridization) of 2–5 nm found in meteorites and interstellar dust [23]. Recently, nanodiamonds have been produced synthetically in the form of films and powders. A large quantity of nanodiamond powder has been successfully synthesized by the detonation of explosive carbonaceous mixture. In fact, carbon atoms in nanodiamonds do not have a purely diamond structure, rather they have an intermediary structure with sp 2and sp 3character, with a diamond‐like core covered by an outer shell of graphitic/amorphous carbon ( Figure 4.3). High‐temperature annealing (>1750 °C) of nanodiamonds could transform them into “carbon nano‐onions” ( Figure 4.3a). Annealing at lower temperatures gives rise to intermediate sp 3@sp 2core–shell structures. The outer graphitic layer is amenable to functionalization and doping, which has been exploited to prepare metal‐free catalysts in several reactions. Moreover, nanodiamonds have been used as metal catalyst support, and the following paragraphs describe more in detail some of the few preparations of catalysts supported on nanodiamonds. As explained, annealing of nanodiamonds at increasing temperatures increases the thickness of the graphitic shell, ultimately transforming them into onion‐like carbon (OLC). By exploiting this concept, some researchers prepared intermediate nanodiamond core/graphitic shells (ND@G) and OLC. Pt nanoparticles (<2 nm) were deposited by incipient wetness impregnation on both supports, and Pt/ND@G exhibited higher turnover rate in CO catalytic oxidation compared to Pt/OLC and Pt/Al 2O 3[25]. Moreover, they were tested in propane dehydrogenation at 600 °C. Under this demanding reaction condition, Pt/ND@G was shown to be significantly more stable than Pt/Al 2O 3,and its stability was attributed to the donation of electron density from the support to the metal (strong metal–support interaction [SMSI]), leading to less crystalline nanoparticles. Due to SMSI, sintering of the metal is prevented and formation of coke is decreased due to the enhanced desorption of propylene. One of the disadvantages of nanodiamonds is that they tend to form aggregates (up to 200 nm). Aggregation can be overcome by dispersing and stabilizing nanodiamonds on a support such as the formation of hybrids with graphene [26].
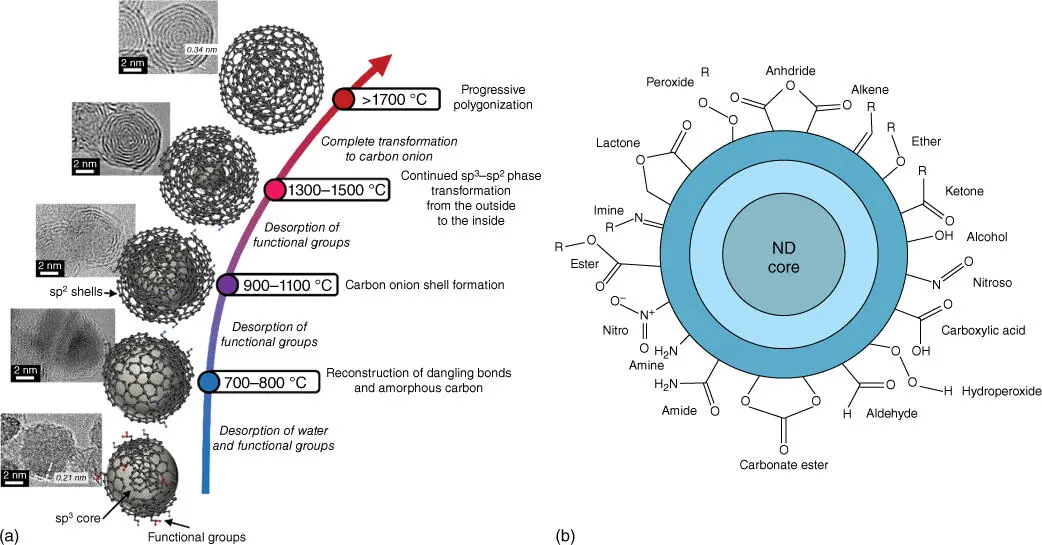
Figure 4.3 (a) Gradual transformation of nanodiamond to onion‐like carbon at increasing annealing temperatures. (b) Schematic representation of nanodiamond.
Source: Zeiger et al. 2016 [24]. Reprinted with permission of Royal Society of Chemistry.
4.2.4 SACs on Carbon Nitrides and Covalent Triazine Frameworks
In graphene doped with heteroatoms (N, O, S, P, etc.), the doped element can function as an anchoring site for metal or metal oxide, leading to the synthesis of hybrid organic–inorganic materials with high stability as a result of the strong binding between the metal species and dopant atoms. Graphitic carbon nitride (g‐C 3N 4) belongs to the family of carbon nitride compounds with a general formula near to C 3N 4(albeit typically with nonzero amounts of hydrogen) and two major substructures based on heptazine and poly(triazine imide) units ( Figure 4.2D). Graphitic carbon nitride is usually prepared by polymerization of cyanamide, dicyandiamide, or melamine. Depending on reaction conditions, carbon nitride exhibits different degrees of condensation, properties, and reactivities. On the other hand, covalent triazine frameworks (CTFs) are structurally related to polymeric carbon nitride ( Figure 4.2E). CTF is a high‐performance polymer framework based on triazine with regular porosity and high surface area. It can be obtained by dynamic trimerization reaction of simple, economical, and abundant aromatic nitriles in ionothermal conditions. Primarily, these materials are large bandgap semiconductors, but their bandgaps are tailorable. For this reason, they are being investigated for photocatalysis. In addition, the loading of metal catalyst should be feasible, due to the presence of the abundant nitrogen atoms and voids within the structure. In fact, they are outstanding supports for SACs because they can stabilize metal ions or small metal nanoparticles even under harsh reaction conditions.
Читать дальше



