Enzyme action and cascades
Enzymes can be divided into classes according to the reactions they catalyze ( Table 2.1). In endocrinology, they frequently operate in cascades where the product of one reaction serves as the substrate for the next. The most simplistic representation of an enzymatic reaction is a physical interaction between the substrate and the enzyme at the latter’s ‘active site’. This proximity catalyzes a molecular modification of the substrate into the product. The product has less affinity for the active site and is released. Other macromolecules can also bind to the enzyme outside the active site and function as co‐factors, adding more complex regulation to the biochemical reaction.
Table 2.1 Definition and classification of enzymes
| Definition |
| An enzyme is a biological macromolecule – most frequently a protein – that catalyzes a biochemical reaction |
| Catalysis increases the rate of reaction, e.g. the disappearance of substrate and generation of product |
| Enzyme action is critical for the synthesis of hormones derived from amino acids and cholesterol |
| Classification |
| Enzyme |
Catalytic function |
Example (and relevance) |
| Hydrolases |
Cleavage of a bond by the addition of water |
Cytochrome P450 11A1/cholesterol side‐chain cleavage (CYP11A1; an early step in steroid hormone biosynthesis) |
| Lyases |
Removal of a group to form a double bond or addition of a group to a double bond |
Cytochrome P450 17α‐hydroxylase/17–20 lyase (CYP17A1; step in the synthesis of steroid hormones other than aldosterone) |
| Isomerases |
Intramolecular rearrangements |
3β‐Hydroxysteroid dehydrogenase/δ‐4,5‐isomerase isoforms (HSD3B; a step in the synthesis of many major steroid hormones) |
| Oxidoreductase |
Oxidation and reduction |
11β‐Hydroxysteroid dehydrogenase isoforms (HSD11B; inter‐conversion of cortisol and cortisone) |
| Ligases or synthases |
Join two molecules together |
Thyroid peroxidase (TPO; a step in the synthesis of thyroid hormone) |
| Transferases |
Transfer of a molecular group from substrate to product |
Phenolethanolamine N ‐methyl transferase (PNMT; conversion of norepinephrine to epinephrine) |
Patients can present with many endocrine syndromes because of loss of enzyme function. For instance, gene mutation might lead to substitution of an amino acid at a key position of an enzyme’s active site. The three‐dimensional structure might be affected so significantly that the substrate is no longer converted to product. In the enzyme cascade that synthesizes cortisol, such mutations can cause various forms of congenital adrenal hyperplasia (CAH) ( Chapter 6). Understanding the biochemical cascade allows accurate diagnosis as the product is lacking while the substrate builds up and can be measured, e.g. by immunoassay or mass spectrometry ( Chapter 4).
Synthesizing hormones derived from amino acids
The amino acid tyrosine can be modified by sequential enzyme action to give rise to several hormones ( Box 2.4). The precise synthetic pathways for dopamine and catecholamines are described in Chapter 6(Figure 6.11) and for thyroid hormones in Chapter 8(Figure 8.4). Melatonin is important in circadian rhythms and is linked to type 2 diabetes. It is generated from the amino acid tryptophan via synthesis of the neurotransmitter, serotonin.
Box 2.4Hormones derived from tyrosine
Thyroid hormones: sequential addition of iodine and coupling of two tyrosines together ( Chapter 8)
Adrenomedullary hormones: hydroxylation steps and decarboxylation to form dopamine and catecholamines ( Chapter 6)
Hypothalamic dopamine formed by hydroxylation and decarboxylation ( Chapter 5)
Synthesizing hormones derived from cholesterol
Steroid hormones are generated by enzyme cascades that modify the four‐carbon ring structure of cholesterol ( Figure 2.6). The precise sequence of enzyme action determines which steroid hormones are generated ( Box 2.5). In addition, cholesterol is a critical building block of all mammalian cell membranes and the starting point for synthesizing vitamin D, which functions, and can be classified, as a hormone ( Chapter 9). Cholesterol is acquired in approximately equal measure from the diet and de novo synthesis mostly in the liver. Dietary cholesterol is delivered to cells as a complex with low‐density lipoprotein (LDL–cholesterol). Intracellular uptake is via the cell‐surface LDL receptor and endocytosis ( Figure 2.3b). De novo biosynthesis commences with coenzyme A (CoA) synthesized from pantothenate, cysteine and adenosine, and proceeds via hydroxymethylglutaryl coenzyme A (HMG‐CoA) and mevalonic acid ( Figure 2.5). The rate‐limiting step is the reduction of HMG‐CoA by the enzyme HMG‐CoA reductase. Pharmacological inhibition of this enzyme to treat hypercholesterolaemia and lessen cardiovascular disease has led to the most widely prescribed drug family in the world (the ‘statins’) and the award of the Nobel Prize to Michael Brown and Joseph Goldstein ( Table 1.2).
Box 2.5Hormones derived from cholesterol
Cholesterol from the diet and from de novo synthesis (mostly in the liver) used to synthesize:
Vitamin D ( Chapter 10)
Steroid hormones:Adrenal cortex: aldosterone, cortisol and sex steroid precursors ( Chapter 6)Testis: testosterone ( Chapter 7)Ovary and placenta: oestrogens and progesterone ( Chapter 7)
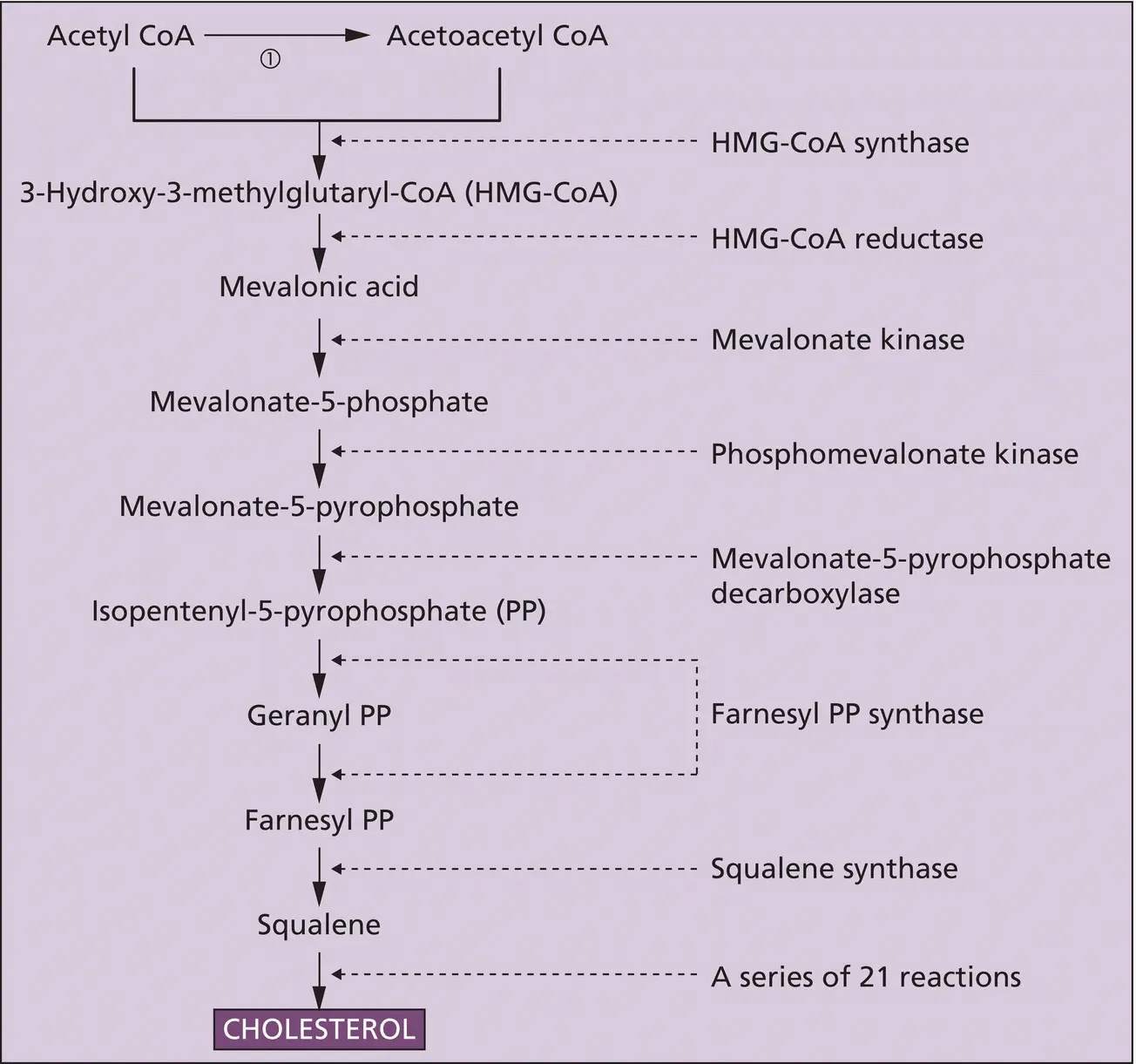
Figure 2.5 Synthesis of cholesterol. Step ① is catalyzed by the enzyme thiolase; otherwise, the enzymes are shown to the right of the cascade. The individual steps between squalene and cholesterol have been omitted.
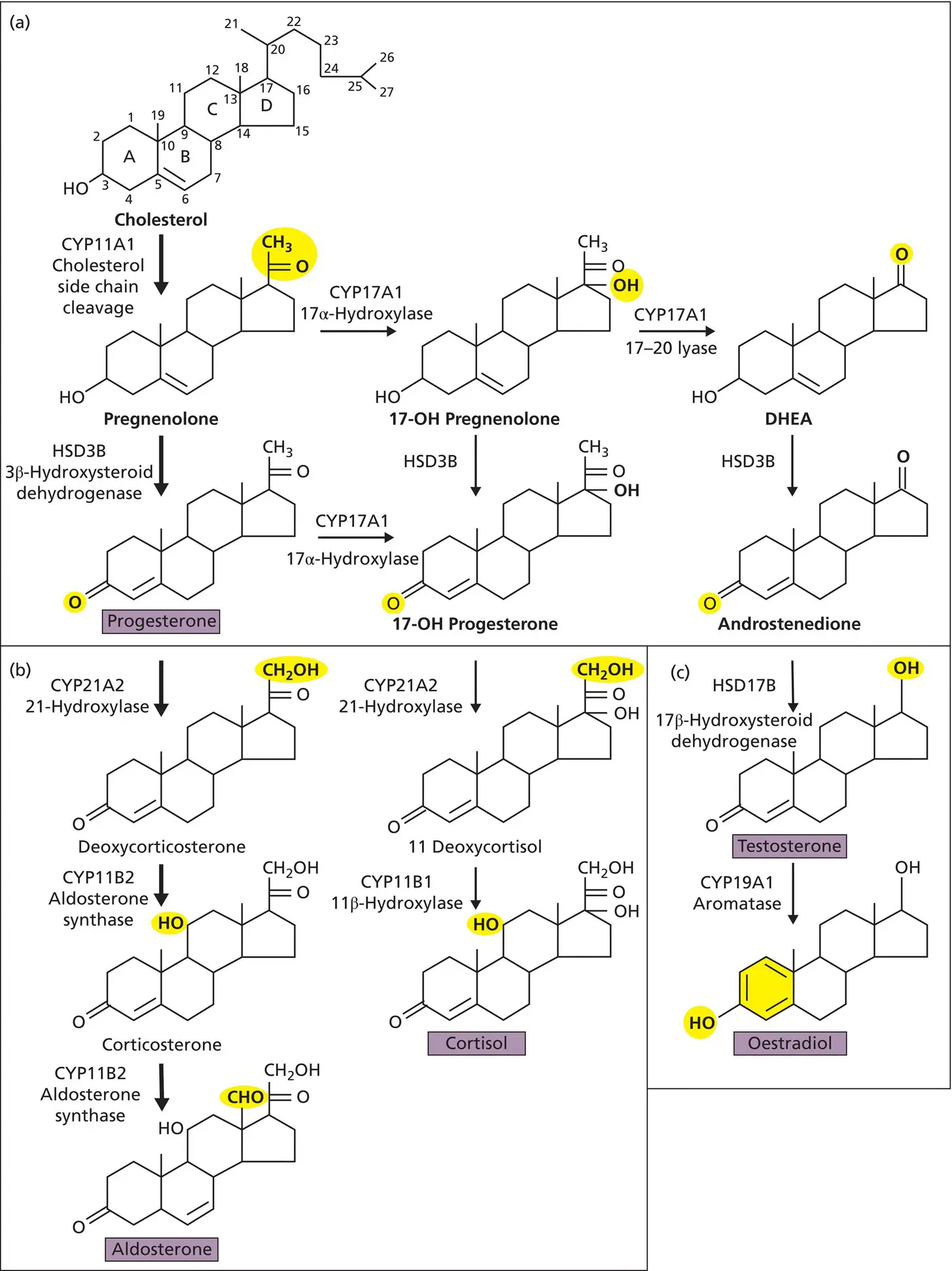
Figure 2.6 Overview of the major steroidogenic pathways. Yellow shading indicates the enzymatic change since the last step. The enzymes are shown by proper name and common name according to their action. Note some enzymes perform multiple reactions (e.g. CYP17A1 acts as both a hydroxylase and lyase) and some reactions are performed by multiple enzyme isoforms (e.g. HSD3B exists as HSD3B1 and HSD3B2). For 17‐OH progesterone, the enzymatic change illustrated is that catalyzed by HSD3B activity rather than CYP17A1. The simplified pathways are grouped into three blocks: (a) common to both the adrenal cortex and the gonad; (b) adrenocortical steroidogenesis; and (c) pathways characteristic of the testis (testosterone) or ovary and placenta (oestradiol). OH, hydroxy; DHEA, dehydroepiandrosterone.
In steroidogenic cells, cholesterol is largely deposited as esters in large lipid‐filled vesicles ( Figure 2.3b). Upon stimulation, cholesterol is released from these stores and transported into the mitochondria, a process that is facilitated by the steroid acute regulatory (StAR) protein in the adrenal and gonad and by the related protein, start domain containing 3 (STARD3), in the placenta. The first step in the synthesis of a steroid hormone is the rate‐limiting conversion of cholesterol to pregnenolone. Pregnenolone then undergoes a range of sequential modifications in the mitochondria or the ER to produce the wide range of steroid hormones.
Читать дальше














