According to Rufino et al. [32], ion exchange, precipitation dissolution, counter‐ion association, and electrostatic interaction are some of the chief mechanisms that govern metal–biosurfactant binding in the contaminated environment. Studies reveal that the complex formation capability of the biosurfactant with the metal ions is the chief cause for their usefulness in metal ion remediation. Precisely, ionic bonds formed in between metal ions and anionic biosurfactants lead to a generation of stronger stabilizing forces as nonionic complexes form, which, of course, are stronger as compared to metal and soil interaction. Because of the neutral charge of the complex with a subsequent amalgamation of the metal into micelles, the complex form of metal–biosurfactants desorb from the soil matrix and move into the soil solution. A detailed study of the proposed mechanism reveals that either an outer‐sphere surface complex formation occurs in between negatively charged surfaces and metals due to strong electrostatic attraction or an inner‐sphere surface complex formation is established between the metal ions and biosurfactant molecules due to chemical bonding in which hydroxide groups serve as ligands. The mechanism of metal binding through both pathways is smoothed in the presence of water molecules and easy protonation and deprotonation of oxide functional groups. The mechanism of metal–biosurfactant interaction is represented in Figure 4.1.
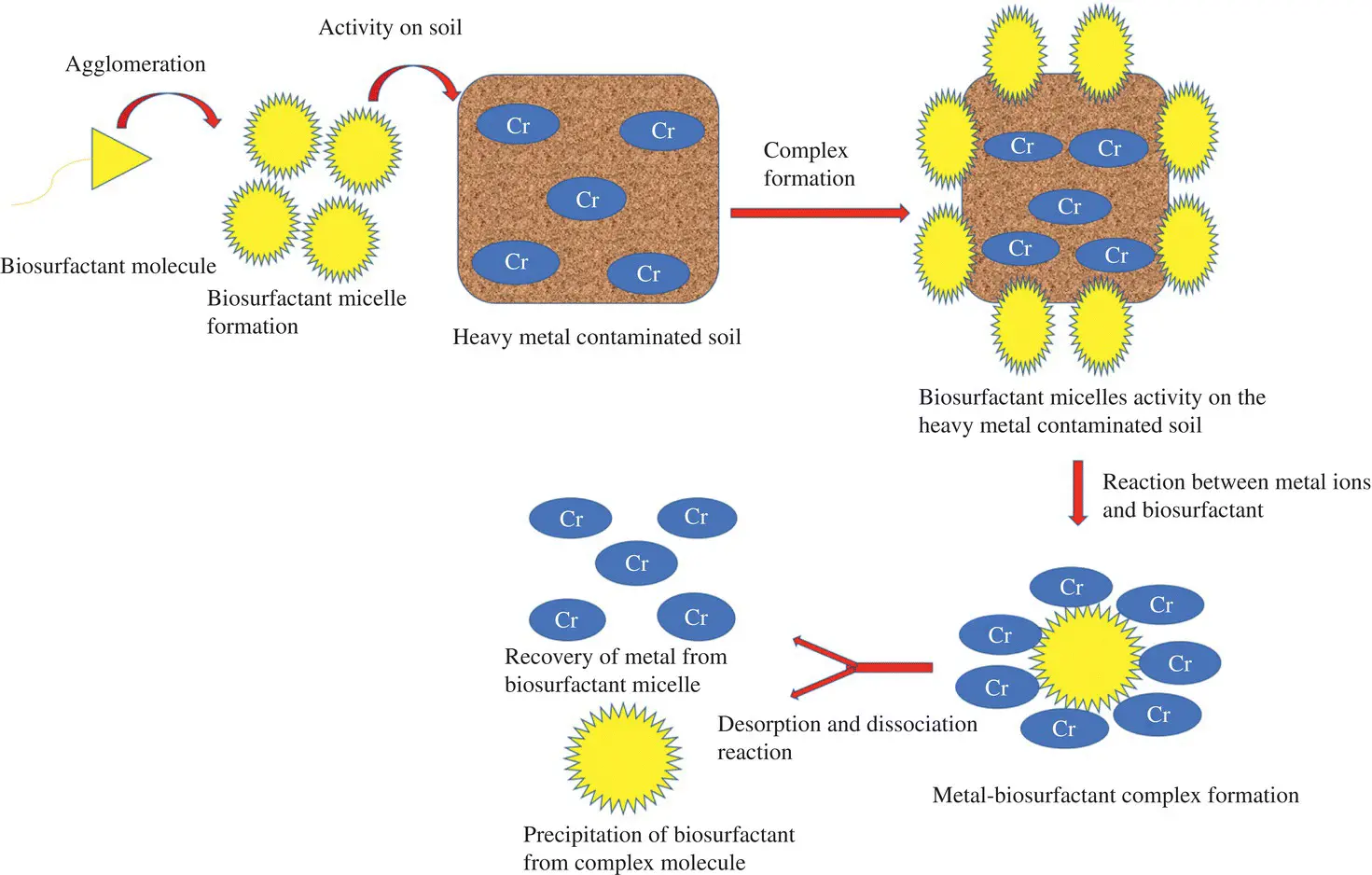
Figure 4.1 Biosurfactant mediated heavy metal remediation.
4.4 Substrates Used for Biosurfactant Production
Microorganisms are identified as the most important source for production of biosurfactants. Willumsen and Karlson [33] in their study found that many of the biosurfactant‐producing microorganisms are hydrocarbon degraders. The proficiency of microbial biosurfactant in the bioremediation as well as in the enhanced oil recovery have been researched extensively [34]. The verities of substrate used for common biosurfactant production is represented in Figure 4.2.
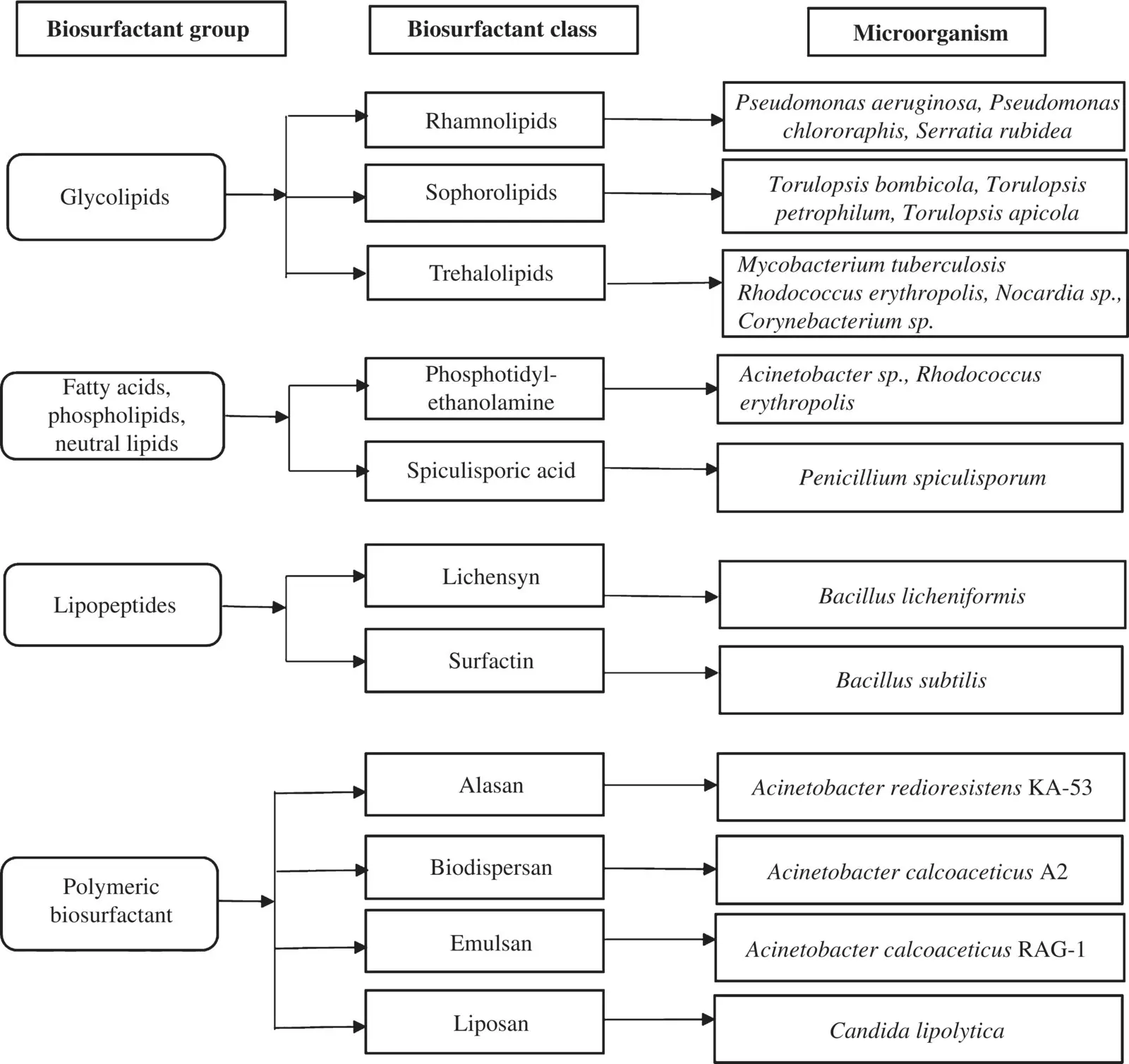
Figure 4.2 Classification of biosurfactants and the respective producing microorganisms.
4.4.1 Biosurfactants of Bacterial Origin
In the growth medium, the hydrocarbons are emulsified by ionic surfactants excreted by some of the bacteria and yeast. Pseudomonas sp. that produce rhamnolipids (RLs) and Torulopsis sp. that are mainly involved in the production of sophorolipids are some examples of these groups of biosurfactants [35, 36].
Some bacterial species have the ability to alter their cell membrane structure by producing some nonionic or lipopolysaccharide biosurfactants. Examples of some nonionic trehalose corynomycolates producing bacterial strains are: Rhodococcus erythropolis, Arthrobacter sp., and various Mycobacterium sp. [37]. Acinetobacter sp. produce lipopolysaccharides, such as emulsan, and Bacillus subtilis produces extensive quantities of lipoproteins, such as surfactin and subtilisin [38, 39]. Table 4.1depicts biosurfactants produced by various strains of bacteria.
Table 4.1 Biosurfactants derived from bacteria.
| Bacteria |
Biosurfactant |
| Serratia marcescens |
Serrawettin |
| Rhodotorula glutinis, Rhodotorula graminis |
Polyol lipids |
| Rhodococcus erythropolis, Corynebacterium sp. Mycobacterium sp., Arhtrobacter sp., Nocardia erythropolis |
Trehalose lipids |
| Pseudomonas sp., Thiobacillus thiooxidans , Agrobacterium sp. |
Ornithine lipids |
| Pseudomonas fluorescens , Leuconostoc mesenteriods |
Viscosin |
| Pseudomonas aeruginosa , Pseudomonas chlororaphis , Serratia rubidea |
Rhamnolipids |
| Pseudomonas fluorescens , Debaryomyces polmorphus |
Carbohydrate‐lipid |
| Pseudomonas aeruginosa |
Protein PA |
| Lactobacillus fermentum |
Diglycosyl diglycerides |
4.4.2 Biosurfactanats of Fungal Origin
Only a few species of fungi are identified for the production of biosurfactants in comparison to bacterial species. Some of the typical fungal strains explored for the production of biosurfactants, as investigated by researchers are, Candida bombicola [40], Candida ishiwadae [41], Candida lipolytica [42], Candida batistae [43], Aspergillus ustus [44], and Trichosporon ashii [45]. The best part of these fungal strains is that they have produced biosurfactants using low‐cost raw materials as their growth substrate. Glycolipids and sophorolipids are one of the most important class of biosurfactants produced by these fungal strains. The various biofactants produced by fungi are shown in Table 4.2.
Table 4.2 Biosurfactants derived from fungi.
| Fungi |
Biosurfactant |
| Torulopsis bombicola |
Sophorose lipid |
| Candida bombicola |
Sophoro lipids |
| Candida lipolytica |
Protein‐lipidpolysaccharide complex |
| Candida lipolytica |
Protein‐lipidcarbohydrate complex |
| Candida ishiwadae |
glycolipid |
| Candida batistae |
sophorolipids |
| Aspergillus ustus |
Glycolipoprotein |
| Tichosporon ashii |
sophorolipids |
4.5 Classification of Biosurfactants
Origin and composition are the two main factors on the basis of which the classification of biosurfactants has been performed. According to Rosenberg and Ron [46], based on the molecular weight, biosurfactants are categorized into two types. The first one is comprised of those compounds that have low molecular weight compounds with lower surface and interfacial tensions and the second one is comprised of high molecular weight compounds with strong surface binding capacity. The majority of low molecular weight biosurfactants comes under the glycolipids, lipopeptides, and phospholipids category while high molecular weight ones are mainly particulate and polymeric surfactants [47]. Another basis for biosurfactant classification is the presence and type of charge on individual polar moiety. The negatively charged surfactants, i.e. anionic usually have a sulphonate or sulfur group as the chief functional group on their cell surface while positively charged or cationic surfactants mainly possess an ammonium and hydroxyl group. Also, surfactants with a neutral or non‐ionic nature are identified and are the products of a 1, 2‐epoxyethane polymerization reaction. When both positively and negatively charged functional groups are present on the same surfactant molecules, they are identified as amphoteric surfactants [48].
4.6 Types of Biosurfactants
There have been so many forms of biosurfactants and each have a common microbial origin. Some of the broad categories of biosurfactant are now discussed.
Glycolipids are the most common type of biosurfactants and consist of mono‐, di‐, tri‐, and tetrasaccharides. The saccharides include glucose, mannose, galactose, glucuronic acid, rhamnose, and galactose sulphate. Some of the microorganisms usually have the same fatty acids and phospholipid composition [49, 50]. Carbohydrates in combination with long‐chain aliphatic acids or hydroxyaliphatic acids are the key component of glycolipids [26]. According to Karanth et al. [51], rhamnolipids, trehalolipids, and sophorolipids are the best‐known glycolipids.
Читать дальше














