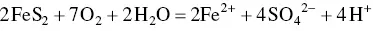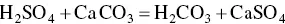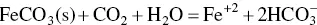No health‐based guideline value is suggested by WHO (2011), however Cl −concentration exceeding the 250 mg/L may lead to a noticeable taste (WHO 2011). But it has been reported that high salinity in irrigation water can damage the crops, affect the plant growth, reduce the soil fertility, and deteriorate the water quality. High chloride may harm the aquatic life through leaf burn, defoliation in sensitive crops, and disturbing the oxygen distribution in water, as well as increasing the metal concentration in water, although health‐related issues are not critically observed yet. However, high sodium water can pose heart disease and high blood pressure, predominantly in vulnerable entities.
Distillation, membrane technology, including reverse osmosis and microfiltration, ion exchange, and treatment using hydrotalcite are generally applied to remediate the high salinity (Grützmacher et al. 2013).
Geogenic sources of sulphate are sulphur‐bearing minerals (e.g. gypsum, anhydrite, and pyrite (Grützmacher et al. 2013). Naturally occurring sedimentary rocks containing sulphur are pyrite (FeS 2) and gypsum (CaSO 4.2H 2O) (Berner 1987; Gourcy et al. 2000). Oxidative weathering of pyrite occurring in alluvial sediments is also one of the causes of high SO 4 2−in water (Raju et al. 2011).
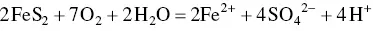
Sulphuric acid reacts with calcium carbonate found in weathered zones and produces calcium sulphate, given by the following reaction:
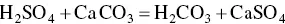
Factors influencing the concentration of sulphate are sulphate‐rich minerals and reducing condition in the aquifer material (Grützmacher et al. 2013)
SO 4concentration above 1000 mg/L can cause purgative effects (WHO 2011).
A natural method for removing sulphate is an anaerobic reduction in constructed wetlands. Other technical methods include reverse osmosis, N.F., and ion exchange (Grützmacher et al. 2013).
Heavy metal is a term given to a division of metals and metalloids that are differentiated by high atomic weight and specific density, five times higher to water or greater than 4000 kg/m 3(Hashim et al. 2011; Kura et al. 2018). Heavy metals are toxic even at deficient concentrations and harmful to human health (Madhav et al. 2020). The following heavy metals are addressed as geogenic pollutants in this chapter.
Iron is the second‐most abundant metallic element in the Earth's outer crust, but its concentration in water is usually low. It occurs in many oxidation states, such as 0, +2, +3, and +6. Many mixed‐valence compounds, like magnetite and prussian blue, have both Fe (II) and Fe (III) centres (Hashim et al. 2011). Igneous rock minerals such as pyroxenes, the amphiboles, biotite, magnetite, and especially the nesosilicate olivine are rich in iron content. In these minerals, iron mostly exists in the ferrous form (Fe 3+) but ferric (Fe 3+) form also occurs, as in magnetite, Fe 3O 4. The ferrous polysulfides like pyrite, marcasite, and the less constant species such as mackinawite and greigite might exist in the presence of sulphur and when reducing conditions prevail. Siderite (FeCO 3) may form when sulphur is less plentiful, whereas under oxidizing environments, ferric oxides or oxyhydroxides species like haematite, Fe 2O 3, goethite, FeOOH, or other minerals having these compositions generally occur (CGWB 2014).
In soil, it is commonly present in organic waste and as plant debris. The oxidation intensity and pH firmly control the chemical nature of iron and its solubility in water. The interaction amid oxidized iron minerals and organic matter or dissolution of FeCO 3leads to a higher concentration of iron in groundwater. This type of water is clear when withdrawn, but soon it becomes cloudy and then browns due to precipitation of Fe (OH) 3(Hashim et al. 2011; Achary 2014a). Organic matter removes the dissolved oxygen within the sediments which creates reduced conditions in which iron‐bearing minerals (siderite/marcasite) has higher solubility causing enriching of the dissolved iron in the groundwater (Applin and Zhao 1989).
The dissolution of Fe in groundwater strongly depends on the concentration of dissolved oxygen and also on the pH of the water to a lesser extent. In groundwater, it generally occurs in two forms as Fe 2+and as Fe 3+. Iron occurs as Fe 3+when the concentration of dissolved oxygen in groundwater is greater than 1–2 mg/L and as Fe 2+when dissolved oxygen is in low concentration. At normal pH of water, Fe 2+is soluble, and Fe 3+is insoluble. At low pH and dissolved oxygen, iron (also manganese) will dissolve readily in groundwater. Aquifers having higher depth and rich in organic matter typically contain less dissolved oxygen. Decomposition of the organic matter leads to depletion of the oxygen in the water, which results in the dissolution of the iron as Fe 2+. In the oxygen‐deprived water, when pumped to the surface, a rust‐coloured iron mineral forms due to the reaction of dissolved iron and the oxygen (CGWB 2014).
In India, groundwater from a large number of areas is highly contaminated with iron. These areas include west Bengal, Rajasthan, Orissa, Goa, Haryana, Jammu and Kashmir, Andhra Pradesh, Karnataka, and Kerala (Garduño et al. 2011). Higher Fe constituent in the coastal aquifers may be due to the interface of oxidized Fe minerals and organic matter and subsequent dissolution of Fe 2CO 3at a moderately lower pH.
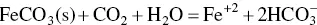
The brown colour of groundwater after extraction from the aquifers is the primary sign of iron presence. The high concentration of Fe in water causes awful taste, staining, turbidity, and equipment problem in the water supply.
Permissible limit of Fe in drinking water prescribed by WHO (2011) and BIS (2012) is 300 μg/L. The use of contaminated groundwater having iron concentration above the permissible limit is highly objectionable as it causes health disorders. The reported health disorders due to Fe contaminated groundwater are disorders of the skin, digestive, respiratory and nervous system, kidney, spinal cord, and heart, as well as mental imbalance, miscarriage, and cancer (Achary 2014b). Deficiency of iron causes anaemia, whereas prolonged consumption of drinking water with a high concentration of iron may lead to a liver disease called hemosiderosis. The water with high iron concentration may seem brownish because of the precipitation of ferric hydroxide and taste astringent. The USEPA (US Environmental Protection Agency) maintains that though drinking water having iron may be consumed safely, iron‐bearing sediments may comprise trace impurities or harbour bacteria that might be damaging. Iron has nutritional value for human beings as it plays an important role in the formation of haemoglobin protein, enzymes, and also used in cellular metabolism. Lesser storage of iron in the body causes the iron deficit, anaemia, fatigue, and affects the immune system. Iron deficiency in children negatively disturbs mental growth, resulting in irritability and concentration ailment. Chronic consumption of surplus amounts of iron results in an ailment termed iron overload, which occurs due to gene mutation. Iron overload, if untreated, can lead to haemochromatosis, a severe illness that could harm the body's organs. Early symptoms of haemochromatosis are fatigue, weight loss, and joint pain; when not cured appropriately it can cause heart disease, liver complications, and diabetes (Garduño et al. 2011; CGWB 2014; Duggal et al. 2017).
Читать дальше