1 ...7 8 9 11 12 13 ...29 PHA synthase (PHAc) is the primary enzymes which polymerizes R‐3‐hydroxyacyl‐CoA precursors. For this all the carbon metabolism is shifted to form R‐3‐hydroxyacyl‐CoA thioester. PHA synthase is broadly classified into four classes. Class I consists of one type of PHAc, while class II contains two type of synthases PHAc1 and PHAc2. Classes II and IV consist of PHAc‐PHAe and PHAc‐PHAr units, respectively (Pötter and Steinbüchel, 2005). Classes I, III, IV favor SCL‐PHA, while Class II favors MCL‐PHA synthesis. Ralstonia eutropha is a model organism for PHA production as it accumulates polymer in nutrient‐deficient condition. Its wild type only synthesizes SCL‐PHA and has to be modified for tailor‐made PHAs (Pohlmann et al., 2006). R. eutropha is engineered to produce copolymer poly(3‐hydroxybutyrate‐ co ‐3‐hydroxyhexanoate) (PHBHHx) from palm oil. Engineered strain contains PHAc from Rhodococcus aetherivorans , enoyl‐CoA hydratase gene (PHAj) from Pseudomonas aeruginosa , and acetoacetyl‐CoA reducatase gene (PHAb). PHAj accumulates PHA, while PHAb alters the level of hydroxyhexanoate (Budde et al., 2011). Since R. eutropha grows slowly and difficult to lyse engineering E. coli is better option. E. coli is not a natural producer of PHAs, as it does not have PHA synthase gene. The first heterologous production of polyhydroxybutyrate (PHB) in E. coli was done in 1988 (Schubert et al., 1988). PHB synthase gene from R. eutropha was cloned in E. coli . The engineered E. coli is able to synthesize PHB but lack the ability to accumulate it.
Increasing the number of PHA synthase gene copies in an organism only increases the PHA levels occasionally that too when the cell dry weight increases. This indicates the importance of cell dry weight over additional copies of PHA synthase gene and shown by many researchers (Tombolini et al., 1995; Huisman et al., 1992; Huisman et al., 1991). E. coli binary fission pattern is changed to multiple fission, by deleting the genes minC and minD (related to binary fission) and overexpressing the genes ftsQ, ftsL, ftsW, ftsN, and ftsZ (related to cell division) (Wu et al., 2016a). This results in more dry weight and more PHB accumulation. Apart from the cell dry weight, cell shape also matters. Along with the deletion of minC and minD gene, deleting gene envC and nlpD which degrades peptidoglycan layer leads to filamentous shape and accumulated 70% PHB (Wu et al., 2016b). All carbon source getting converted to biomass and no product is not beneficial industrially. Therefore, a glucose‐sensing toggle switch is created that automatically senses glucose limitation and activates product formation leading to shorter growth phases and good titers of PH (Bothfeld et al., 2017).
There is no doubt that genetic engineering increases the production of PHAs in many aspects. But the substrate used in PHA production is costly, and unusual substrates (whey, lipid, molasses, glycerol, etc.) are not directly converted to PHA. Whey contains 4.5% of lactose, engineering Cupriavidus necator which is the natural producer of PHA so that it can grow on lactose. For this purpose, lac operon consisting of lac I, lac Z, lac O from E. coli is inserted into C. necator (Povolo et al., 2010). The lac operon is inserted within the depolymerase gene (phaZ1) which reduces the depolymerization of the polymer and increase in its accumulation. E. coli which naturally utilizes lactose but do not have PHA synthase gene is engineered with PHA synthase gene from C. necator . The recombinant strain was grown in cell recycle fed‐batch culture giving 87% PHB content (Ahn et al., 2001). Molasses, which is the predominant waste produced by sugar industry, contains almost 50% sucrose. Microorganisms should be engineered with sucrose utilization genes in order to use molasses as the carbon source for PHA production. One such gene is sucrose permease (csc) of E. coli which has been engineered in C. necator to produce PHBHHx using sucrose as a sole carbon source (Loo et al., 2005). Glycerol is the major waste of soap industry by fat hydrolysis and is now also being generated from biodiesel manufacturing. Aerobic metabolism gene from E. coli , glycerol kinase, aquaglyceroporin, FAD‐dependent glycerol 3‐phosphate dehydrogenase are transformed in C. necator to obtain PHB accumulation (Fukui et al., 2014).
Organic acids are widely distributed in nature from ages and have been used by us in our daily life be it lactic acid (in curd) or citric acid (in fruits like lemon, orange, etc.), indicating them to be the building block chemicals. Most of the organic acids can be produced microbially or as an intermediate compound. These microorganisms can be wild type or improved genetically to increase the yield. Products produced by microorganisms are of high purity, selectivity, and are cost efficient (Singh et al., 2016). Organic acids have a wide range of applications and its market is expected to grow at a rate of 60% till 2021. Though the direct demand of the particular organic acids in the market is low but can be created by chemical industry (Sauer et al., 2008). For example, maleic anhydride can be replaced by succinic acid if there is no price limitation. There are number of products that can be derived from succinic acid which includes butanediol, butyrolactone, tetrahydrofuran, bionolle, etc. Bionolle is the polymer of succinic acid and has property similar to polyethylene.
Their applications are in food, beverages, pharmaceuticals, textile, detergents, solvents, petrochemicals, dyes and adhesives, rubber, perfumes, and plastics, etc. If we talk about gluconic acid then gluconic acid and its component have a variety of applications. Gluconic acid is used to prevent milk stone formation in dairy industry and to clean aluminium cans. Various salts of gluconic acid like sodium, calcium, and iron are used as additive in cement, supplement in calcium and iron deficiency, respectively (Ramachandran et al., 2006).
Citric acid, succinic acid, lactic acid, and gluconic acid are discussed below with their structure shown in Figure 1.3.
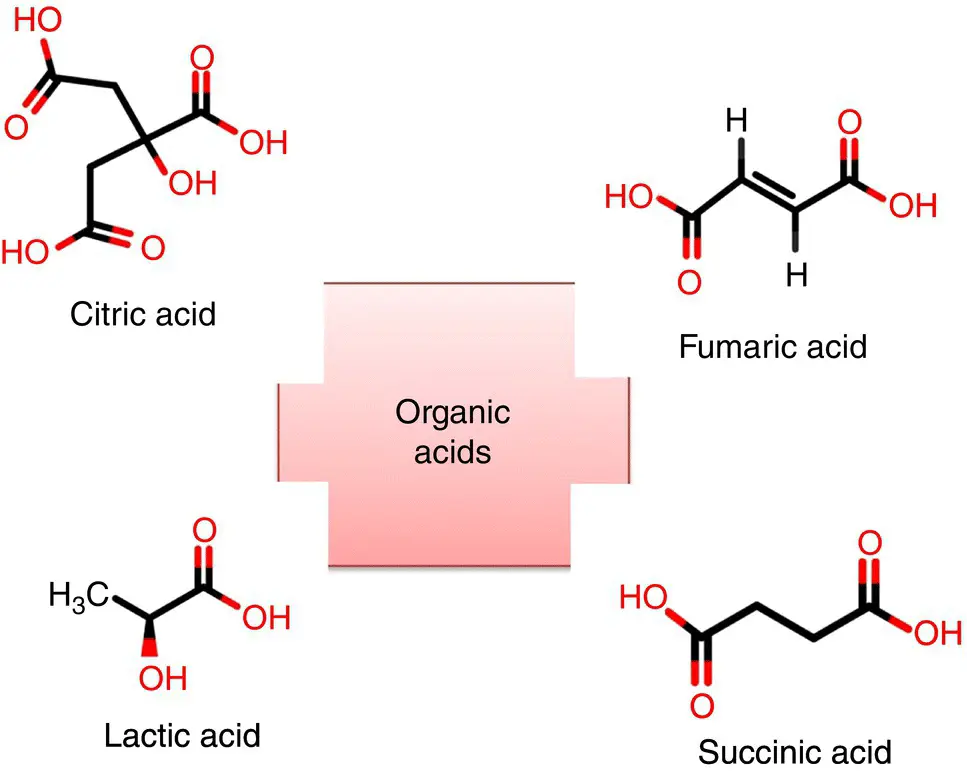
Figure 1.3 Different organic acids with their structures.
Citric acid (2‐hydroxy‐propane‐1,2,3‐tricarboxylic acid) is colorless, odorless, in its pure form, and is water soluble. It is widely distributed in plants and animals and is omnipresent in nature as the intermediate compound in krebs cycle or tricarboxylic acid cycle. It is used as flavoring agent in food industry (Soccol et al., 2006). It has a major role in pharmaceutical industry as a blood preservative, in chemical industry as antifoam agent, and in soap industry as stabilization of fats and oils (Max et al., 2010). Its estimated production capacity is approximately 2 million tons per year (Steiger et al., 2017). Citric acid synthesis pathway along with other organic acids synthesis is shown in Figure 1.4.
It was first discovered in lemon (citrus fruit) and now about 99% of citric acid is produced from microbial fermentation. Wehemer (1893) discovered the production of citric acid by Penicillium glaucum , but could not scale up the process (Vandenberghe et al., 1999). In 1917, strains of Aspergillus niger were found to synthesize citric acid and soon became the method of choice for industrial production of citric acid (Chen and Nielsen, 2016). Highest producing strains of Aspergillus can accumulate 200 g/l citric acid under optimum conditions (Steiger et al., 2017). For improved production rate, metabolic and genetic engineering of Aspergillus strains are done which can tolerate low pH levels and can secrete excess citric acid in the medium (Ruijter et al., 1997; Steiger et al., 2019). Carbon source utilization plays an important role in industrial production of citric acid. Despite giving higher yields glucose cannot be used industrially as it is not cost efficient. Therefore, liquified corn starch, glycogen, etc. are used. Using liquified corn starch leads to the production of isomaltose with the action of enzyme glucosidase and cannot be used for citric acid production. There is another enzyme glucoamylase that releases glucose from starch and is beneficial. So, Wang et al., (2016) deleted the glucosidase encoding gene and overexpressed glucoamylase gene in A. niger . This leads to the decrease in residual sugar to about 88.2% and increase in citric acid production to 16.9%, reaching up to 185.7 g/l.
Читать дальше













