However, no simple relationship governed differences between atomic weights in Mendeleyev’s table, whereas Moseley’s new classification—“the law of Moseley,” as Rutherford later called it—provided a ladder with ninety-two regular rungs. It was beautifully simple and has provided the basis for physical and chemical analysis of atomic structure ever since. [12] Hydrogen, the smallest atom with its one orbiting electron and a charge of one on the nucleus, occupies the first place, helium with its doubly charged nucleus and two, orbiting electrons is in place number two and so on until uranium with its ninety-two whizzing electrons.
At the end of his work, Moseley had no remaining doubt that his findings supported Bohr’s theories and said so firmly in the papers he published.
By identifying that there were gaps in his table, Mendeleyev had turned it into a tool for the prediction of new elements. By 1886, three with the chemical properties he had identified—scandium, gallium, and germanium—had been discovered. Moseley’s “law” suggested that between hydrogen at number one and uranium at ninety-two, there were still seven elements (whose characteristics were predicted) as yet undiscovered. Moreover, Moseley’s classification placed several element-pairs in their correct order in the periodic table, whereas Mendeleyev, in order to get the chemical properties to fit, had had to place them out of sequence in his ranking by atomic weights.
At the same time, however, there was a difficulty. Moseley’s tabulation left no room at the upper, heavier end of the range for the recently identified products resulting from radioactive decay, like some discharges from radium and thorium. While working at McGill, Rutherford and Soddy had argued that such products were elements in their own right. If so, it had to be possible to fit them into the table.
The anomaly was resolved by Frederick Soddy, who identified the “Law of Radioactive Displacements” revealing the existence of “isotopes.” Soddy deduced that elements could exist in several forms, identical in their chemical and most of their physical properties but differing in their atomic weight. To name them, he borrowed two words from ancient Greek— isos, meaning “the same,” and topos, meaning “place”—to signify that isotopes of the same element occupied the same place in the table of chemical elements. Others had also been moving toward the same conclusions, which were an integral part of the jigsaw puzzle of the atom being assembled with such rapidity.
• • •
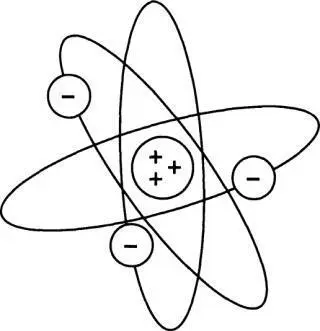
The Rutherford-Bohr atomic model
In the spring of 1914 Rutherford, convinced by the accumulating weight of evidence, put his own considerable weight firmly behind the Rutherford-Bohr model of the atom. This was also the year when, on 12 February, the forty-two-year-old Rutherford was knighted by the king. Sir Ernest reacted to the honor with due modesty but was plainly delighted, reveling in his costume of velvet breeches, cocked hat, sword, and silver buckles. Former pupils from around the world wrote to congratulate him. One of them was the German chemist Otto Hahn, who had studied under Rutherford at McGill and would one day play a critical part in the discovery of nuclear fission.
Hahn was born in Frankfurt in 1 879, the son of a prosperous artisan. Rejecting his father’s suggestion that he become an architect, he instead studied organic chemistry. He was, by his own admission, a “slightly superficial, easygoing” young man, not a hard worker. In his final school report, two of his three top marks were for gymnastics and singing. At Marburg University, he enjoyed “beery days” and once dueled with sabers. However, in 1904, a chance event changed his life. As preparation for working in industry, Hahn went to London to learn English. By sheer good fortune, he managed to get a place at University College, in the laboratory of Sir William Ramsay.
Hahn at this time knew nothing of radioactive substances, but Ramsay set him to extracting radium from barium salt. Somewhat to Hahn’s surprise, this task led him to the discovery of a radioactive substance, radiothorium. He watched the material glowing in his darkroom, where he was sometimes distracted by a female assistant who found excuses to join the personable young man in the gloom, though, as he later wrote, “I never dared to kiss her.” He was very fond of women, but his English sometimes let him down in the chase. Once, while dancing the fashionable two-step at a university ball, he whispered conversationally in his partner’s ear: “You, here in England, you dance on the carpet. We in our country prefer to dance on the naked bottom.” The girl left the dance floor.
Fascinated by his new area of work, Hahn abandoned thoughts of industry. Instead, he wrote to Rutherford, then in Montreal, believing him to be “the only person who had real grasp” of the new science. Rutherford agreed to take Hahn for six months. He enjoyed life in the “New World,” although the discovery that the Rutherford household was teetotal was a shock. He sought solace in his pipe, lending his “much-chewed specimens” to Rutherford, who frequently mislaid his own. Hahn admired Rutherford’s directness, even his simple way of dressing. When a photographer arrived to take Rutherford’s photograph, Hahn had to lend him some detachable cuffs because he had not bothered to put any on. More than anything, though, Hahn had found his vocation.
He returned to Germany in 1906 to the Institute of Chemistry in Berlin and began working on the sample of radiothorium Ramsay had given him as a parting gift. He was joined the following year by a slight, dark-haired theoretical physicist from Vienna, Lise Meitner, who would earn from Einstein the accolade of “the German Marie Curie.” She had arrived in Berlin to research under Max Planck and been immediately drawn to the confident, energetic, easygoing Hahn. They decided to work together on radiation experiments, but the institute’s director, Emil Fischer, had barred women from the premises. His pretext, after an incident involving a wild-haired Russian student and a Bunsen burner, was that he feared they would set their hair alight. However, he allowed Lise Meitner to work with Hahn in a room that had formerly been the carpenter’s workshop and had its own entrance from the street. When she needed to use the toilet, she had to visit a nearby restaurant.
Lise Meitner’s difficulties reveal how extraordinary Marie Curie’s achievements had been and the scale of the problems then facing women scientists. Meitner was one of just thirty women working in the new field of radioactivity between 1900 and 1910. She was such a rarity that even Rutherford, who encouraged women in his own laboratories, committed a gaffe. Passing through Berlin in 1908 after receiving his Nobel Prize, he was introduced to the thirty-year-old Lise Meitner. He had seen her name in publications, but even “Lise” had failed to alert him. He exclaimed, “in great astonishment: ‘Oh, I thought you were a man!’”
• • •
In the period leading up to the First World War, Rutherford’s ability and personality had made him the hub of the international scientific community. When hostilities began in the summer of 1914, he was shocked and depressed. Believing that science should know no boundaries, he did his best to maintain contacts with colleagues overseas. He also worried about what would happen to his “boys,” as he called his current and former students, whether foreign, like Hans Geiger, by then back in Germany, or British, like James Chadwick, whom the outbreak of war left stranded in Berlin.
Читать дальше













