After the establishment of Hayflick limit and as a result of numerous studies, it was found that normal fibroblasts in culture retain a diploid karyotype and have a limited life expectancy. In addition, normal cells lack oncogenic potency. These requirements to cultures of normal cells were issued in the form of normative documents.
If these requirements are met, it is possible to use human fibroblasts cultivated outside the body for the production of immunobiological prepartions, and then for therapeutic purposes. Scientific research and clinical developments in this area are very intensive, due to general rise of cellular technologies based on stem cells.
Currently, there are two main approaches to the treatment of skin with preparations containing live fibroblasts. On the one hand, methods and preparations for the treatment of skin defects due to wounds and burns became widely known with the help of cultures of allogenic (from another organism) embryonic fibroblasts. An alternative to these methods is the possibility of using autologous (a person’s own) human fibroblasts for cell replacement therapy of the skin (Zgursky, 2004).
It is believed that the best results of cell therapy are obtained by using cultures of embryonic fibroblasts, which have a greater proliferative potential than cultures from adult donors. However, the use of cells derived from embryos has a number of limitations, including ethical ones. At the same time, data accumulate that a person’s own fibroblasts retain their potential during aging, despite a decrease in their number in the aging organism (Terekhov, 1984).
To achieve primary results, it is necessary to obtain fibroblast cultures from adult donors. It was found that proliferative abilities of the fibroblasts obtained (in comparison with embryonic skin fibroblasts) are sufficient to use them for cell therapy (Zgursky, 2004).
Methods of obtaining and cultivation of autologous skin fibroblasts
Cell material to obtain a culture of autogenous fibroblasts is taken by biopsy of the skin (a small section of the skin with a size of about 0.5 cm 2) in the forearm or in the ear area of the patient with local anesthesia.
Obtaining and cultivation of fibroblasts from skin biopsy are carried out according to the standard procedure. To obtain the primary culture of cells, the tissue is washed with a physiological solution containing antibiotics, placed in a sterile Petri dish and treated with a solution containing trypsin or collagenase (both are enzymes). Under the influence of this solution, the tissue softens, and living cells (fibroblasts) spread from it to the bottom of a dish. Then the dish is filled with nutritive growth medium (see. below) and placed in a CO 2 incubator (special tightly closed box that maintains a constant temperature of 37 0C and an increased concentration (5—7%) of carbon dioxide in the air (Fig. 6); the usual concentration of CO 2 in the atmosphere is 0.03—0.04%, in which fibroblasts cannot grow). Cells begin to multiply and after filling the whole bottom of a dish, the fibroblasts are removed from the growth surface with a mixture of solutions: Versene (solution of EDTA-ethylene diamine tetraacetic acid and inorganic salts) and trypsin. The primary cultured cells are centrifuged, washed from the solution components and suspended in the culture medium.
Next, the cells are transferred into culture flasks (Figures 7a, 7b), containing liquid growth medium.



Growth medium contains a set of amino acids, glucose and others nutrients, as well as 10—20% fetal calf serum containing the necessary growth factors for cell division. The flasks themselves are placed in the CO 2 incubator. Flasks do not closed tightly, so that the air with a high content of carbon dioxide from the incubator could flow into the flasks. It is possible also to use flasks with ventilated lids (with built-in sterile filter), through which an exchange with air in the incubator is performed. This method provides conditions for the growth (division) of cells.
All works are carried out in strictly sterile conditions in a laminar flow hood (Figures 8a, 8b), in which the fan pumps sterile (free from bacteria) air through the sterilizing air filter, located at the back or top of the box, with a small additional pressure.
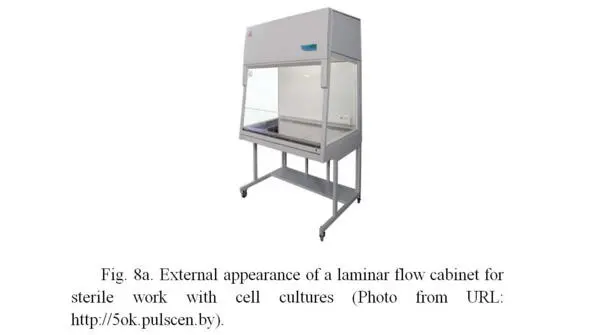

Due to excessive pressure inside the laminar hood, the external air with bacteria does not penetrate inside the box, so cell cultures remain sterile. The work inside the laminar hood is carried out by a highly qualified specialist using sterile laboratory glassware, including flasks, pipettes, Petri dishes, etc. Additional sterility is carried out by firing the surfaces of flasks neck, pipette working parts in the flame of an alcohol or gas burner, which is installed inside the laminar hood and is turned on constantly during the entire period of work with biological material. The specialist himself is outside the laminar hood, but all actions are carried out by hands in sterile surgical gloves inside the hood through an open window, from which the sterile air constantly leaves, what does not allow the non-sterile air (with bacteria) from the outside.
Cell cultures obtained from patients are used to develop the necessary number of cells sufficient for procedures (6—8 passages or cell divisions in the culture). In further procedures the fibroblasts are tested for absence of contamination (infection) of HIV/AIDS, hepatitis A, B and C, mycoplasma and chlamydia. Testing is carried out using PCR (polymerase chain reaction) and immunoassay methods. The lack of oncogenic potential of cells is determined on athymik (non-immune) mice. Next, a monolayer culture of cells is removed from the surface of the flasks with a mixture of solutions of Versene and trypsin and suspended (stirred) using serological pipettes in sterile saline for injection. Then the cells centrifuged and washed 2—3 times from Versene and trypsin in the same saline. The final suspension is stored at +4 0C. Cell preparations are used within 24 hours after obtaining the suspension.
The viability of cells in the suspension is determined by establishing the culture after different periods of incubation and counting the number of attached cells, as well as their ability to form colonies.
The procedure of cell therapy
The procedure for administration of cells to the patient is carried out by means of tunneling injections for large wrinkles or multiple injections for small wrinkles.
The number of cells in the preparations varies depending on the procedure, the volume of the preparation administered is on average of 1—3 ml per procedure. The number of injected cells is on average from 1 to 10 million per procedure.
The method of administration of cells is shown in figures 9a, 9b. Cells are injected using an insulin syringe into the dermal (middle) layer of the skin (Zgurski, 2004). The needle of insulin syringe is very thin, so the procedure causes minimal discomfort.
Читать дальше

















